Chemistry Extra Test Prep Examples UNIT: Moles, % Comp, EF
advertisement

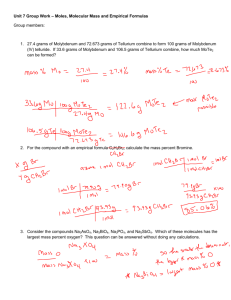
Chemistry Extra Test Prep Examples UNIT: Moles, % Comp, EF & MF 1. What is the mass (in grams) of 0.0714 mol of iron (III) sulfate, Fe2(SO4)3? 2. What is the percent composition of the compound (NH4)3P. 3. Vitamin C contains 40.92% C, 4.58% H, and 54.50% O. What is the empirical formula for vitamin C? 4. A certain compound has an empirical formula of C3H4. If the molar mass of this unknown is 121 grams/mol, what is the molecular formula for this compound? 5. Fred the Chemist is in the lab trying to find the empirical formula of a compound made from magnesium and chlorine. Use the data below to help out Fred: Mass of crucible Mass of crucible + Mg Mass of crucible + product (Mg + Cl) Answers on second page 20.00 grams 20.45 grams 21.76 grams 1. Ans: 28.6 grams Fe2(SO4)3 2. Ans: 49.38% N; 14.21% H; 36.40% P 3. Ans: C3H4O3 4. Ans: C9H12 5. Ans: MgCl2