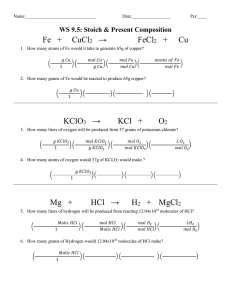
Combustion Analysis Chemistry Problems
advertisement

Combustion Analysis Problems (Challenge) 1. You should be able to set up and solve chemical analysis of an unknown compound by combustion problems. 7.321 g of an organic compound containing carbon, hydrogen, and oxygen was analyzed by combustion. The amount of carbon dioxide produced was 17.873 g and the amount of water produced was 7.316 g. Determine the empirical formula of the compound. 2. 0.1101 grams of an organic compound containing carbon, hydrogen, and oxygen was analyzed by combustion. The amount of carbon dioxide produced was 0.2503 grams and the amount of water produced was 0.1025 grams. A determination of the molar mass of the compound indicated a value of approximately 115 grams/mol. Determine the empirical formula and the molecular formula of the compound. 3. 2.012 g of a metal carbonate, MCO3, is heated to give the metal oxide and 0.855 g CO2. MCO3 (s) MO (s) + CO2 (g) What is the identity of the metal?