Tertiary Compounds
advertisement

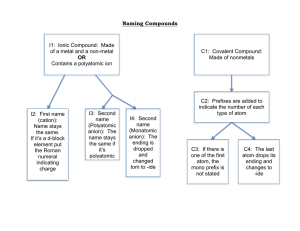
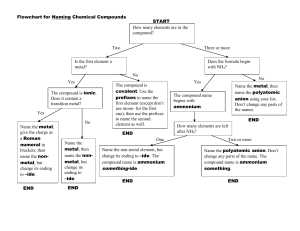
Name ______________________________________________________ Period ____________ Chapter 7/9: Nomenclature & Properties Keller/Rosenzweig Tertiary Compounds Polyatomic Ions: 1. Write the formula for the following tertiary ionic compounds using the following rules: 1. 2. 3. 4. Identify and write in the charges of the cation and polyatomic ion in the compound Put parentheses around the polyatomic ion Criss-cross the charges Reduce subscripts OUTSIDE the parentheses if necessary (do not reduce the subscripts of the polyatomic ion inside the parentheses – it must stay as is!!) a. lithium sulfate b. calcium nitrate c. copper(II) permanganate d. strontium hydroxide e. chromium (III) carbonate f. manganese (IV) sulfite g. barium chlorate **h. ammonium sulfate 2. Name the ionic compound using the following rules: 1. Name the cation 2. Name the polyatomic ion a. NaNO3 b. Ca(CN)2 c. Mg3(PO4)2 d. Al(OH)3 e. ZnSO4 f. Cs2CO3 g. KClO3 **h. NH4C2H3O2 3. Name the ionic compounds that are made from transition metals using the following rules: 1. Write in the charge for the anion you are sure of 2. Work backwards to find the charge of the cation 3. Name the cation and anion 4. Include the roman numeral in the name a. CuS b. FeP c. NiI3 d. Cr(MnO4)4 e. Pb(NO3)2 f. Pb(SO4)2 g. Cu3(PO4)2 h. Cu3PO4 Practice: Determine whether each compound below needs a roman numeral. Then name the compound. COMPOUND ROMAN NUMERAL? Y or N CuI2 Mn(OH)4 Na2SO4 NiCO3 Write the formula for each compound in the chart below: NAME FORMULA manganese (VI) hydroxide chromium (III) fluoride zinc sulfate aluminum oxide tin(IV) phosphate NAME