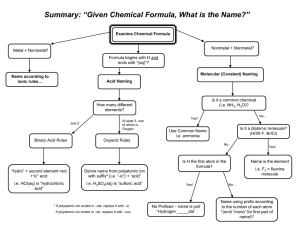
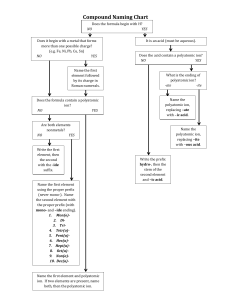
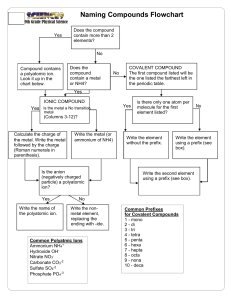
General Rules for Naming Compounds Does the compound involve a metal? Yes No Are both non-metals the same? Is the metal a multivalent metal? Yes Write the name of the metal, write the Roman Numeral indicating its charge (look at the nonmetal/polyatomic ion charges to figure out the charge) Write the name of the metal (NO roman numerals) It’s a diatomic element Is there a polyatomic ion? Yes No Look up the polyatomic ion(s) in the table and name using normal metal nonmetal rules (NO prefixes) What is the other half of the compound? Non-Metal No Yes No Use the prefix naming system: Don’t include mono for the first element Polyatomic Ion Write the name of the non-metal second, but change the ending to “ide” Look up the polyatomic ion in the table Special Case: If Hydrogen is the first element – use acid naming! Formula Writing ➢ ➢ ➢ ➢ Ionic Compounds Determine the charges on each ion (from either periodic table, or table of polyatomic ions) Criss-cross the charges Reduce if possible Check your answer