Naming Type IV (ACIDS
advertisement

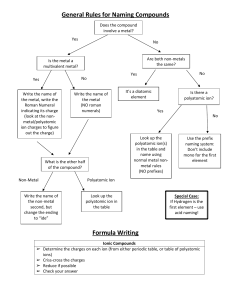
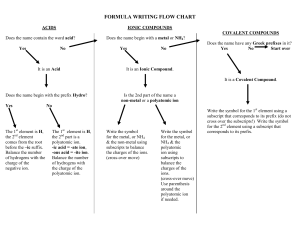
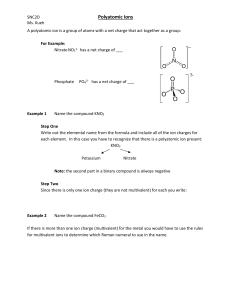
Naming Type IV (ACIDS) Name____________________________________________Period______ This type of naming is used when a compound starts with a H. With the exception of H2O (water) and H2O2 (hydrogen peroxide) Chemical Formula: These are acids. 1. Always balance the charges so they add to equal zero. H+1 Y-3 3(+1) + 1(-3 )= 0 2. The number of each needed becomes the subscript. Don’t write 1’s. H3Y Chemical Name: 1. If the acid is a binary acid – just two elements where one is an H, then name it like this hydro(base name)ic acid. 2. If the acid is an oxy-acid, in other words a polyatomic and a hydrogen, then the hydrogen just indicates that this is an acid and the naming comes from the polyatomic name. If the polyatomic ion name ended in: - ate then with a H the ending changes to -ic acid. (Think thanksgiving dinner – I ate too much and I got sick) - ite then with a H the ending changes to - ous acid (Think fight takes two of us) Positive ion Negative ion Chemical Formula Chemical Name Hydrochloric acid H3PO4 Sulfurous acid NO3 CO3 Your Turn! (Make sure to write in all the charges of all the ions!) Positive Ion 1 2 3 4 5 6 7 8 9 10 Negative Ion S Chemical Formula HF Perchloric acid H2SO4 NO3 H3PO3 Carbonic acid Bromic acid HNO2 SO3 11 HClO2 12 13 14 15 Chemical Name chlorous acid SO2 H3BO3 Hydroiodic acid Positive Ion Negative Ion Chemical Formula 16 Hypochlorous acid 17 18 Chemical Name HCN S 19 Dichromic acid 20 Oxalic acid 21 H2SiO4 22 Permanganic acid 23 H2CrO4 24 Silicic acid 25 HNO2 26 Br 27 IO3 28 29 HIO2 [Fe(CN)6]3- Ferrocyanic acid 30 Thiosulfuric acid 31 H2SeO4 32 Hydroiodic acid 33 C6H7O7- Citric acid 34 C3H5O3- Lactic acid 35 C7H5O2- Benzoic acid 36 C7H5O3- Salicylic acid 37 HCl 38 HBr 39 HI 40 HF