and PSII
advertisement

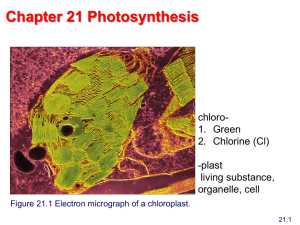
Chapter 21 Photosynthesis Biochemistry by Reginald Garrett and Charles Grisham Essential Question 1. How is solar energy captured and transformed into metabolically useful chemical energy? 2. How is the chemical energy produced by photosynthesis used to create organic molecules from carbon dioxide? Outline 1. What are the general properties of photosynthesis? 2. How is solar energy captured by chlorophyll? 3. What kinds of photosystems are used to capture light energy? 4. What is the molecular architecture of photosynthetic reaction centers? 5. What is the quantum yield of photosynthesis? 6. How does light drive the synthesis of ATP? 7. How is carbon dioxide used to make organic molecules? 8. How does photorespiration limit CO2 fixation? The Sun - Ultimate Energy 1.5 × 1022 kJ of sunlight energy falls on the earth each day • 1% is absorbed by photosynthetic organisms and transformed into chemical energy – CO2 fixation: 6CO2 + 6H2O → C6H12O6 + 6O2 – 1011 tons of CO2 are fixed globally per year – Formation of sugar from CO2 and water requires energy • The remaining 99%, 2/3 is absorbed by earth and ocean; 1/3 is lost as light reflected back 21.1 What Are the General Properties of Photosynthesis? • Photosynthesis occurs in thylakoid membranes of chloroplasts – Structures involving paired folds (lamellae) that stack to form "grana" – The soluble portion of the chloroplast is the "stroma" – The interior of the thylakoid vesicles is the "thylakoid space" or "thylakoid lumen" • Chloroplasts possess DNA, RNA and ribosomes Photosynthesis Consists of Both Light Reactions and Dark Reactions • The light reactions – Associated with the thylakoid membranes – Capture light energy and convert it to chemical energy in the form of reducing potential (NADPH) and ATP, with evolution of oxygen • The dark reactions (CO2 fixation) – Located in the stroma – Use NADPH and ATP to drive the endergonic process of hexose sugar formation from CO2 in a series of reactions in the stroma Photosynthesis Consists of Both Light Reactions and Dark Reactions Figure 21.4 The light-dependent and light-independent reactions of photosynthesis. Water is the Ultimate e- Donor for Photosynthetic NADP+ Reduction The reaction sequence for photosynthesis in green plants: 2 H2O + 2 NADP+ + x ADP + x Pi → O2 + 2 NADPH + 2 H+ + x ATP + x H2O The stoichiometry of the metabolic pathway of CO2 fixation 12 NADPH + 12 H+ + 18 ATP + 6 CO2 + 12 H2O → C6H12O6 + 12 NADP+ + 18 ADP + 18 Pi A More Generalized Equation for Photosynthesis: CO2 + 2H2A Hydrogen Hydrogen acceptor donor → (CH2O) + 2A + H2 O Reduced Oxidized acceptor donor In photosynthetic bacteria, H2A can be H2S or other oxidizable substrates, like isopropanol: In higher plants, H2A can be H2O and 2 A is O2 21.2 How Is Solar Energy Captured by Chlorophyll? • Chlorophyll is a photoreactive, isoprene-based pigment – A planar, conjugated ring system—similar to porphyrins – Mg2+ is coordinated in the center of the planar conjugated ring structure – A long chain alcohol, phytol, group confers membrane solubility – Aromaticity makes chlorophyll an efficient absorber of light • Light absorption promotes an electron to a higher orbital, enhancing the potential for transfer of this electron to a suitable acceptor Since the absorption spectra for a and b differ, plants that possess both can harvest a wider spectrum of incident energy. Accessory light-harvesting pigments increase the possibility for absorption of light β-Carotene, an accessory light-harvesting pigment in leaves. Phycocyanobilin, a blue pigment found in cyanobacteria. The Light Energy Absorbed by Photosynthetic Pigments Has Several Possible Fates • Each photon represents a quantum of energy • Each quantum of light energy has 4 possible fates: 1. Loss as heat. Energy can be dissipated as heat through redistribution into atomic vibrations within the pigment molecule. 2. Loss as light. A light-excited electron can return to a lower orbital, emitting a photon of fluorescence. 3. Resonance energy transfer. Excitation energy can be transferred to a neighboring molecule. 4. Energy transduction. The excitation energy changes the reduction potential of the pigment, making it an effective electron donor. Figure 21.7 Possible fates of the quantum of light energy absorbed by photosynthetic pigments. Transduction of Light Energy into Chemical Energy Involves Oxidation-Reduction • The basis of photosynthesis is transduction of light energy into chemical energy (of oxidation-reduction). • Photon absorption raises chlorophyll (Chl) to Chl* • Electron transfer from Chl* to an adjacent molecule A, producing oxidized Chl (Chl•+) and reduced A (A-) • Oxidation of A- eventually culminates in reduction of NADP+ to NADPH • The system is restored to its original state once NADPH is formed and water is oxidized Figure 21.8 Model for light absorption by chlorophyll and transduction of light energy into an oxidation-reduction reaction. The process culminates in production of NADPH and oxidation of water. Photosynthetic Units Consist of Many Chlorophyll Molecules but Only a Single Reaction Center • The photosynthetic unit consists of (Fig 21.9) – Several hundred light-capturing chlorophylls – A pair of special chlorophylls in the reaction center • Light is captured by one of the "antenna chlorophylls" and routed from one to the other until it reaches the reaction center chlorophyll dimer that is photochemically active • Oxidation of chlorophyll leaves a cationic free radical, Chl•+, whose properties as an electron acceptor are important to photosynthesis Figure 21.9 Schematic diagram of a photosynthetic unit. 21.3 What Kinds of Photosystems Are Used to Capture Light Energy? Oxygenic phototrophs have two distinct photosystems: PSI (P700) and PSII (P680) • PSI has a maximal red light absorption at 700 nm and use ferredoxins as terminal electron acceptors • PSII has a maximal red absorption at 680 nm and use quinones as terminal electron acceptors • Chloroplasts given light at 680 and 700 nm simultaneously yield more O2 than the sum of amounts when each is used alone • All chlorophyll is protein-bound—as part of either PSI or PSII or light-harvesting complexes (LHCs) PSI and PSII Participate in the Overall Process of Photosynthesis • PSII splits water (photolysis), producing O2, and feeds the electrons released into an electron transport chain that couples PSII to PSI via cytochrome b6f • PSI provides reducing power in the form of NADPH • Electron transfer between PSII and PSI pumps protons for chemiosmotic ATP synthesis • Essentially, electrons flow from H2O to NADP+, driven by light energy absorbed at the reaction centers • Light-driven phosphorylation of ADP to make ATP is termed photophosphorylation Figure 21.10 Roles of the two photosystems, PSI and PSII. The Pathway of Photosynthetic Electron Transfer Is Called the Z Scheme • PSI and PSII contain unique complements of electron carriers, and these carriers mediate the stepwise of electrons from water to NADP+ • According to their standard reduction potential, the zigzag result resembles the letter “Z”, Thus the pathway name – the Z scheme (Fig 21.11a) • Overall potosynthetic electrons is accomplished by three membrane-spanning supramoleculear complex (Fig 21.11b) 1. PSII 2. Cytochrome b6f complex 3. PSI • • • • • Pheo= pheophytin PQ = plastoquinone pool PC = plastocyanin "F"s = ferredoxins Ao = a special chlorophyll a • A1 = a special PSI quinone • Cytochrome b6/cytochrome f complex is a proton pump Figure 21.11 The Z scheme of photosynthesis. Oxygen Evolution Requires the Accumulation of Four Oxidizing Equivalents in PSII (Fig 21.12) • When isolated chloroplasts that have been held in the dark are illuminated with very brief flashes of light, O2 evolution reaches a peak on the third flash and every fourth flash thereafter • The interpretation of this observation: PSII (P680) cycles through five oxidation states • 1 e- and 1 proton are removed in each step • Oxygen (O2) is released in the fifth step and two more molecules of H2O are bound • The net of this cycle: 2H2O oxidized to O2 + 4H+ 3 7 11 15 19 Figure 21.12 Oxygen evolution requires the accumulation of four oxidizing equivalents in PSII Electrons Flow From Pheophytin in the Z Scheme Via Plastoquinones Plastoquinone (PQ) exists as a pool within the chloroplast membrane. Because of its lipid nature, plastoquinone is mobile within the membrane and shuttles electrons from PSII to the cytochrome b6f complex. Plastoquinone A has nine isoprene units and is the most abundant plastoquinone in plants and algae. Note the similarity to Coenzyme Q. Electrons from PSII Are Transferred to PSI via the Cytochrome b6f Complex • The cytochrome b6f complex – is a large multimeric protein possessing 26 transmembrane α-helices – This complex is homologous to the cytochrome bc1 complex of mitochondria – Mediate the transfer of electrons from PSII to PSI and to pump protons across the thylakoid membrane • Plastocyanin (PC in the Z scheme, Figure 21.11) is a small copper-containing protein that carries electrons from cytochrome b6f to PSI • The copper in PC cycles between the reduced Cu+ and oxidized Cu2+ states in this transfer 21.4 What Is the Molecular Architecture of Photosynthetic Reaction Centers? • Rhodopseudomonas viridis is a photosynthetic prokaryote with a single photosystem that resembles PSII – Lack an OEC and the capacity to oxidize water • The reaction center (P870) of R. viridis photosystem is located in the plasma membrane – Is composed of four peptides: L, M, H and cytochrome (Figures 21.14) – L and M each bear two bacteriochlorophyll molecules and one bacteriopheophytin – L also has a bound quinone molecule, QA The R. viridis Photosynthetic Reaction Center is an Integral Membrane Protein Cytochrome subunit Figure 21.14 The R. viridis reaction center (RC). (a) Diagram of RC showing light activation and path of etransfer. Photosynthetic Electron Transfer by the R. viridis Center Leads to ATP Synthesis • The prosthetic groups of the R. viridis reaction center (P870, BChl, Bpheo, and the bound quinones) are fixed in a spatially to facilitate photosynthetic e- transfer (Fig 21.15) • Photoexcitation of P870 leads to e- loss via electron transfer to the nearby bacteriochlorophyll (BChl) • The e- is then transferred via the L protein to QA (on L) and then to QB (on M) • The reduced quinone formed at QB diffuses to a neighboring cytochrome bc1 membrane complex • Oxidation of the quinone at bc1 is coupled to proton transport, and thus eventually to ATP synthesis Figure 21.15 Photophosphorylation. Photoexcitation of R. viridis RC leads to reduction of a quinone, Q, to form QH2. Oxidation of QH2 by the bc1 complex leads to H+ translocation for ATP synthesis. The Molecular Architecture of PSII Resembles the R.viridis Reaction Center • Type II photosystems in higher plants, green algae and cyanobacteria contain more than 20 subunits but resemble the R. viridis center in several respects • T. elongatus PSII is a homodimeric structure, with each monomer having 23 different protein subunits – Each “monomer” has at least 34 transmembrane α-helical segments – The D1 and D2 subunits of PSII are similar to the L and M subunits of the R. viridis reaction center – Electron transfer from the QA site on D2 to the QB site on D1 is analogous to the electron transfer seen in R. viridis Figure 21.16 The molecular architecture of the T. elongatus PSII dimer. (a) the arrows show the direction of electron transfer. How Does PSII Generate O2 From H2O? • The oxidation of H2O to O2 is chemically difficult • The oxygen-evolving complex (OEC) is a large globular protein domain on the lumenal side of the D1 subunit of PSII (Figure 21.16) • The active site of the OEC contains a cube-like metal cluster of four Mn ions, one Ca ion, and five O atoms bridging the Mn atoms. • This cluster is held in place by Glu, Asp, and His residues of D1 and a Glu from CP43, a chlorophyllcontaining inner subunit of PSII (Figure 21.17) • Electron removal from H2O molecules at the cluster (one from each Mn) facilitates O2 formation Figure 21.17 Structure of the PSII oxygenevolving complex (OEC). The Architecture of PSI Resembles the R. viridis Center and also PSII Architecture • PSI from T. elongatus is a cloverleaf-shaped trimer • Each monomer consists of 12 subunits and 127 cofactors • All of the prosthetic groups essential to function are located in just 3 of the subunits: PsaA, PsaB, PsaC • PsaA and PsaB form the reaction center heterodimer • PsaC interacts with the stromal face of the PsaA-PsaB dimer • The electron-transfer system of PSI consists of three pairs of chlorophyll molecules that mediate electron transfer to the quinone acceptor • The T. elongatus quinone is phylloquinone Figure 21.18 The architecture of PSI. (a) Subunit organization. How Do Green Plants Carry Out Photosynthesis? • The structure of a PSI-light-harvesting complex (LHC) supercomplex has been determined • LHC1 is a complex of 16 distinct protein subunits and 200 prosthetic groups • Four LHC1 subunits form an arc around one side of the PSI reaction center • A second LHC (LHC2) binds to another side • The many Chl molecules and other light-harvesting molecules of the supercomplex form an integrated network for highly efficient transfer of light energy into P700 Figure 21.19 View of the plant PSI-LHC1 supercomplex, from the stromal side of the membrane. Chlorophylls are green, lipids are red. 21.5 What Is the Quantum Yield of Photosynthesis? • The quantum yield of photosynthesis is defined as the amount of product formed per equivalent of light input i.e., the amount of O2 evolved per photon absorbed • Four photons per reaction center: 8 quanta total drive the evolution of 1 O2, reduction of 2 NADP+, and the translocation of 14 H+ • Chloroplast ATP synthase has 14 c-subunits, so 3 ATPs are formed for every 14 H+ translocated • So 3 ATP should be synthesized from an input of 8 photons of light 21.6 How Does Light Drive the Synthesis of ATP? Photophosphorylation • The transduction of the electrochemical gradient into the chemical energy of ATP is carried out by the chloroplast ATP synthase, more properly called the CF1CF0-ATP synthase • Electron transfer through the proteins of the Z scheme drives the generation of a proton gradient across the thylakoid membrane • Protons pumped into the lumen of the thylakoids flow back out, driving the synthesis of ATP • CF1-CFo ATP synthase is similar to the mitochondrial ATP synthase Figure 21.20 Mechanism of photophosphorylation. Photosynthetic electron transport establishes a proton gradient that is tapped by the CF1-CF0 ATP synthase to drive ATP synthesis. c-ring of CF1-CF0-ATP synthase Figure 21.21 The c-subunit assembly of spinach chloroplast ATP synthase consists of 14 identical subunits. Cyclic Photophosphorylation Generates ATP but Not NADPH or O2 • Noncyclic photophosphorylation has been described in Figure 21.20 • In cyclic photophosphorylation, the photoexcited electron removed from P700 returns to P700 in a pathway (Figure 21.22) – Cyclic photophosphorylation depends only on PSI, not on PSII – It diverts the activated electron lost from PSI back through the PQ pool, the cytochrome b6f complex, and plastocyanin to re-reduce P700+ Figure 21.22 The pathway of cyclic photophosphorylation by PSI. 21.7 How Is Carbon Dioxide Used to Make Organic Molecules? Fixation of CO2 is a common (though not universal) ability in plants, algae, etc. • Melvin Calvin at Berkeley in 1945 showed that Chlorella could take up 14CO2 and produce 3phosphoglycerate • CO2 was combining with a 5-C sugar to form a 6C intermediate • The 6-C intermediate breaks down to two 3-Pglycerates • Ribulose-1,5-bisphosphate is the CO2 acceptor in CO2 fixation Ribulose-1,5-Bisphosphate Carboxylase Fixation is accomplished by ribulose bisphosphate carboxylase (oxygenase), rubisco • Probably the world's most abundant protein • Rubisco exists in 3 forms – E: inactivative – EC: carbamylated inactivative(CO2 added to Lys201) – ECM: carbamylated and has Mg2+; active • RuBP (substrate!) is inhibitor and must be released from inactive rubisco by rubisco activase. Carbamylation and Mg2+ then activate. E-RuBP activase E EC ECM Ribulose-1,5-Bisphosphate 2-Carboxy-3keto-arabinitol-1,5-Bisphosphate 3-phosphoglycerate The Calvin-Benson Cycle • The set of reactions that transform 3-P-glycerate into hexose sugar • Serve three metabolic purpose 1. The principal CO2 fixation pathway in nature 2. Accomplish the reduction of 3-phosphoglycaerate to glyceraldehyde-3-P, so carbohydrate synthesis becomes feasible 3. Transform 3-C compounds into 4-, 5, 6, and 7-C compounds • A large part of this pathway is really a disguised gluconeogenesis pathway! • With some pentose phosphate pathway reactions thrown in (see Chapter 22 for gluconeogenesis and the pentose-P pathway). CO2 Fixation into Carbohydrate Proceeds Via the Calvin-Benson Cycle Figure 21.25 The CalvinBenson cycle CO2 Fixation into Carbohydrate Proceeds Via the Calvin-Benson Cycle The Carbon Dioxide Fixation Pathway Is Indirectly Activated by Light • The activities of key Calvin cycle enzymes are coordinated with the output of photosynthesis • In effect, these enzymes respond indirectly to light activation 1. Light induces pH changes in chloroplast compartments 2. Light energy generates reducing power 3. Light induces movement of Mg2+ ions from the thylakoid vesicles into the stroma The Carbon Dioxide Fixation Pathway Is Indirectly Activated by Light 1. Light induces pH changes in chloroplast compartments – Rubisco, rubisco activase, and several Calvin cycle enzymes are more active at alkaline pH 2. Light energy generates reducing power – Reduced ferredoxin and NADPH 3. Light induces movement of Mg2+ ions from the thylakoid vesicles into the stroma Figure 21.27 Light-induced pH changes in chloroplast compartments. These pH changes modulate the activity of key Calvin cycle enzymes. Figure 21.28 The pathway for light-induced reduction of Calvin cycle enzymes. Light-generated reducing power (Fdred = reduced ferredoxin) provides e- for reduction of thioredoxin (T) by FTR. 21.8 How Does Photorespiration Limit CO2 Fixation? Ribulose bisphosphate carboxylase/oxygenase catalyzes an alternative reaction in which O2 replaces CO2 as the substrate added to RuBP • This oxygenase reaction diminishes plant productivity because it leads to loss of RuBP (the CO2 acceptor) • The products of the oxygenase reaction are 3phosphoglycerate and phosphoglycolate • Dephosphorylation converts phosphoglycolate to glyoxylate • Transamination yields glycine Figure 21.29 The oxygenase reaction of rubisco. (a) The reaction of ribulose bisphosphate carboxylase with O2 and ribulose bisphosphate yields 3-phosphoglycerate and phosphoglycolate. (b) Conversion of two phosphoglycolates to serine + CO2. The C-4 Pathway for CO2 Fixation The Hatch-Slack Pathway • Not an alternative to Calvin cycle, nor even a net CO2 fixation pathway • It is a CO2 delivery system – Carries CO2 from the O2-rich leaf surface to interior cells where O2 won't compete in the rubisco reaction – Oxaloacetate and malate are the CO2 transporters • Read about Crassulacean acid metabolism, an alternative to the C4 pathway used by succulent plants • Tropical Grasses Use the Hatch-Slack Pathway to Capture Carbon in CO2 Fixation Figure 21.30 Essential features of the compartmentation and biochemistry of the Hatch-Slack pathway of carbon dioxide uptake in C4 plants.