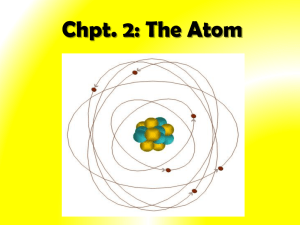
“Plum Pudding” model in class Bohr's Model
advertisement
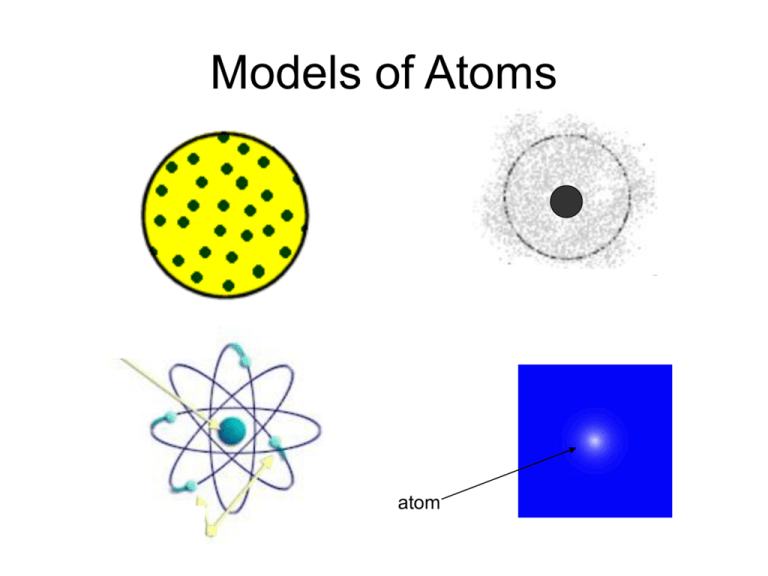
Models of Atoms atom The Electron JJ Thomson used the cathode ray tube to prove that the atom was made up of electrons Click here to listen to him talk about it • • • • But, what was that green light? Was it a light? Was it a particle? To test this, he brought a magnet close to the cathode ray tube to see what would happen. • So, the magnet caused the cathode ray to move. What does that tell us? • Would a magnet affect a light from a flashlight? (you could try this at home) • Probably not. • Therefore, the cathode ray must be a particle! 1897 - J.J. Thompson • Negatively charged electrons stuck into a lump of positively charged material. • Explained some electrical properties • It did not explain number of protons and electrons. Plum-Pudding model Plum Pudding is an English dish sort of like bread pudding with raisins in it. An American analogy to his atom would be… …Is like… But we’ll still refer to it as the “Plum Pudding” model in class Bohr’s Model • “Planetary Model” • Electrons are arranged in circular paths or orbits, around the nucleus. • Electrons move have fixed energy so they don’t fall into the nucleus. Ernest Rutherford In 1908, Rutherford performed the Gold Foil Experiment. Proposed a nuclear atom in which electrons surround a dense nucleus. Thought of the rest of the atom as mostly empty space. (1871-1937) In the experiment, he shot alpha particles (very small, very dense, very fast particles) at a thin layer of gold foil. • He expected all of the alpha particles to go straight through • It would be like if you were shooting bullets at a cake…all of the bullets (or alpha particles) would go straight through the cake (or gold foil atoms) Gold foil Detector screen Alpha particles source Alpha particles Thomson’s Atom Alpha particles Or… Quantum Mechanical Model • Erwin Schrodinger used a mathematical approach to describe an atom. • It estimates the probability of finding an electron. The fuzzy cloud represents an area that there is 90% probability of finding an electron.