Nuclear Transmutations Worksheet: Equations & Reactions
advertisement
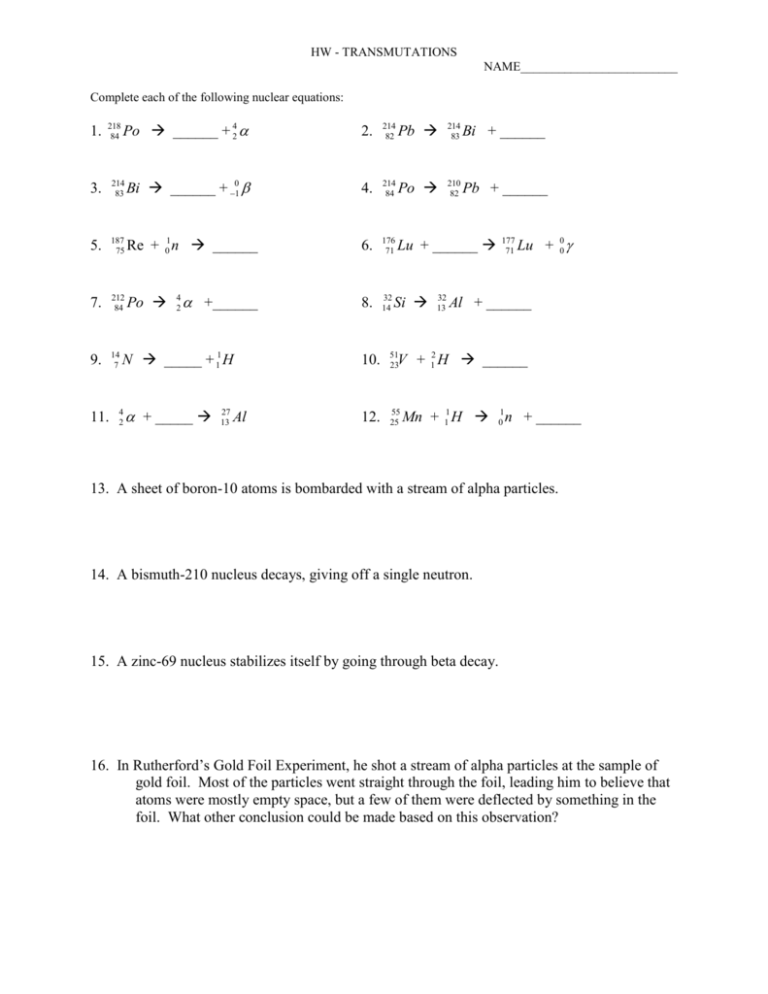
HW - TRANSMUTATIONS NAME_________________________ Complete each of the following nuclear equations: 1. 218 84 Po ______ + 42 2. 214 82 Pb 214 83 Bi + ______ 210 82 Pb + ______ 3. 214 83 Bi ______ + 10 4. 214 84 Po 5. 187 75 Re + 01 n ______ 6. 176 71 Lu + ______ 7. 212 84 Po +______ 8. 32 14 9. 14 7 11. 4 2 Si N _____ + 11 H 10. 51 23 + _____ 12. 55 25 4 2 27 13 Al 32 13 177 71 Lu + 00 Al + ______ V + 12 H ______ Mn + 11 H 1 0 n + ______ 13. A sheet of boron-10 atoms is bombarded with a stream of alpha particles. 14. A bismuth-210 nucleus decays, giving off a single neutron. 15. A zinc-69 nucleus stabilizes itself by going through beta decay. 16. In Rutherford’s Gold Foil Experiment, he shot a stream of alpha particles at the sample of gold foil. Most of the particles went straight through the foil, leading him to believe that atoms were mostly empty space, but a few of them were deflected by something in the foil. What other conclusion could be made based on this observation?