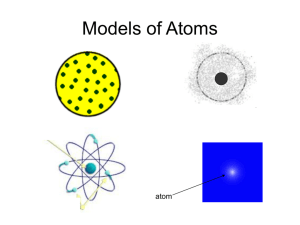
Rutherford's Model of the Atom
advertisement
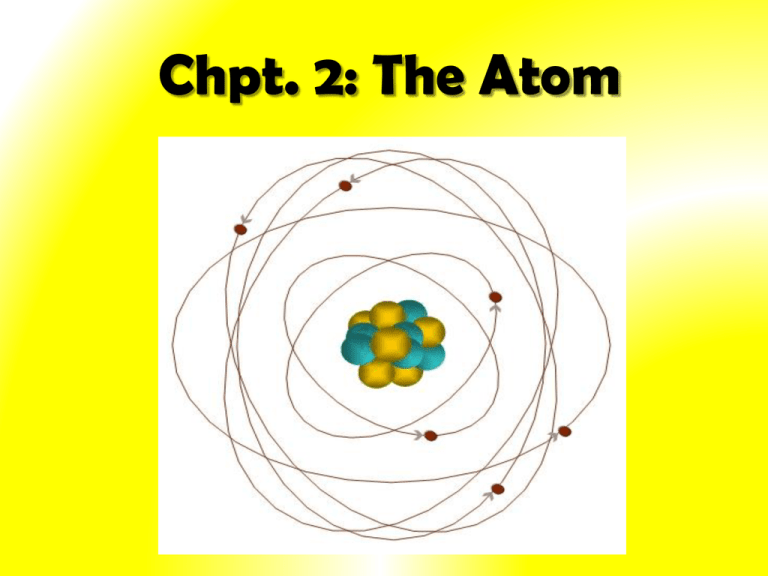
Chpt. 2: The Atom History of the Atom 1. Greek Philosophers (400BC): - first proposed that matter was composed of minute particles - believed that the tiny particles of which all matter was composed were so small that nothing smaller was possible ‘Atomos’ Greek word for indivisible - ATOM 2. John Dalton (1808): Dalton’s Atomic Theory - All matter is made up of very small particles called atoms - All atoms are indivisible. They cannot be broken down into simpler particles - Atoms cannot be created or destroyed What is inside the atom??? Discovery of the Electron 3. William Crookes – cathode ray tube (1875): -, passed electric current through gases at low pressure - invisible radiation that caused the glass to glow came from *cathode (-) - called cathode rays - showed existence of this radiation by placing Maltese Cross inside the tube *Note: Cathode = plate connected to negative end of battery Anode = plate connected to positive end of battery 4. J.J Thomson – cathode ray tube experiments (1897): - devised experiment to investigate if cathode rays consisted of charged particles - cathode rays attracted up towards positive plate (anode) => consisted of negatively charged particles - hence cathodes are streams of negatively charged particles called electrons Definition: cathode rays are streams of negatively charged particles called electrons Thomson’s Experiment Voltage source Passing an electric current makes a beam appear to move from the negative to the positive end Thomson’s Experiment Voltage source By adding an electric field he found that the moving pieces were negative Further experiment: - he found electrons were also deflected in magnetic field - found ratio of charge to mass of the electron (e/m): (electrical charge of electron) (mass of electron) = 1.76 x 108 coulombs = 1 gram of electrons *Note: In 1891 George Stoney proposed that the smallest amount of electric charge be called an electron. Thomson's ‘Plum Pudding Model’ of the Atom (1898): Proposed that since atoms are neutral each one consists of: -- a sphere of positive charge -- electrons embedded randomly Dough = positive charge Raisins = electrons 5. Robert Millikan (1909): - Experiment to measure size of charge on electron – Oil Drop Experiment - Charge of one electron = 1.6 x 10-19 coulomb THUS…. Mass of e- = 9.11 x 10-31g Discovery of radiation led to the use of alpha particles in experiments Alpha particles are positively charged particles produced by certain radioactive substances 6. Ernest Rutherford (1909): Rutherford discovered the nucleus and the proton Rutherford's Gold Foil Experiment - bombarded tin foils of gold with alpha (α) particles - If plum pudding model was correct he expected: The alpha particles to pass through without changing direction very much Fluorescent Screen Gold Foil Lead block Uranium What He Got!!!!! *Note: Detector flashes - of light produced when α particles strike zinc/sulphite screen Rutherford’s Gold Foil Experiment Results • Most alpha particles passed straight through undeflected Explanation/Conclusion • Atom mainly empty space occupied by electrons (negative) • Some were deflected at wide angles • Both the mass and positive charge were concentrated in a small dense core which he called the nucleus • Few deflected back along own path Rutherford – discovery of protons (1924): • Light atoms (oxygen, nitrogen) were bombarded with alpha particles - small POSITIVE charged particles were given off • This did not occur with heavier metals e.g. gold • Explanation – alpha particles were breaking up the nuclei of the lighter atoms to release positively charged particles • referred to these small positive particles as protons 7. James Chadwick (1932): • Search for a neutral particle to cement the nucleus • Bombarded beryllium with alpha particles • Produced neutral particles – neutron Properties of Sub-Atomic Particles Name Relative Charge Relative Mass Location Proton +1 1 nucleus Electron -1 1/1836 (no mass) outside nucleus Neutron 0 1 nucleus Dalton Model of the Atom • Small, indivisible spheres J.J. Thompson’s Model of Atom • Plum Pudding Model, 1896 •Thought an atom was like plum pudding Rutherford’s Model of the Atom • Rutherford Model, 1911 • Thought atom was mostly empty space: - Nucleus - Electrons (negatively charged) revolving around nucleus Bohr’s Model of the Atom Neils Bohr, 1913 • Similar to Rutherford’s model • Thought atom was mostly empty space: - Nucleus in center is dense, positively charge - Electrons in orbits around nucleus (Modern) Quantum Mechanical Model of the Atom Heisenberg, Schrodinger, many others, ~1926 • Think atom is mostly empty space: - Nucleus in center is dense, positively charge - Electrons cannot locate Evidence for the existence of small particles!!! Why is it possible to smell the perfume that someone is wearing from several metres away? Diffusion • The process by which molecules of a substance spread through a solid, liquid or gas. • Some examples which can be demonstrated in the lab:- Gas Jar full of air Demonstration • Diffusion of ink in water • Diffusion of NH3 and HCl • Diffusion of smoke in air Diffusion of NH3 and HCl Diffusion of NH3 and HCl Word Equation: Ammonia + Hydrogen chloride = Ammonium chloride (Gas) (Gas) Chemical Equation: NH3 + HCl (White powdered ring) = NH4Cl