Quantum Applets & Relativistic Paradoxes
advertisement

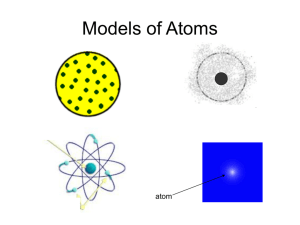
Quantum Applets & Relativistic Paradoxes G. Rothstein Physics/Pre-AP Physics Academics Townview Magnet Center • "If at first the idea is not absurd, then there is no hope for it.” -Albert Einstein J. J. Thomson and The Atom • Believed that a massive, positively charged substance filled the atom • He pictured the electrons arranged within this substance like raisins in a muffin. Ernest Rutherford & The Atom • Rutherford’s team bombarded metal (gold) foil with alpha particles. They measured the deflection of alpha particles directed normally onto a sheet of very thin gold foil. Under the prevailing plum pudding model, the alpha particles should all have been deflected by, at most, a few degrees. However they observed that a very small percentage of particles were deflected through angles much larger than 90 degrees. What are Alpha Particles? • Alpha particles (named after the first letter in the Greek alphabet, α) consist of two protons and two neutrons bound together into a particle identical to a helium nucleus; hence, it can be written as He2+. • Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. What are Alpha Particles? • Alpha particles consist of two protons and two neutrons that act as a single particle. An alpha particle is identical to the nucleus of a Helium atom. When alpha particles are emitted from an unstable radioactive nucleus, the atom is transmuted into a different element. Ernest Rutherford & The Atom • Top: Expected results: alpha particles passing through the plum pudding model of the atom undisturbed. Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated positive charge. • • • • • • • • Observations Most of the alpha particles pass straight through the gold foil. Some of the alpha particles get deflected by very small amounts. A very few get deflected greatly. Even fewer get bounced of the foil and back to the left. Conclusions The atom is 99.99% empty space. The nucleus contains a positive charge and most of the mass of the atom. • The nucleus is approximately 100,000 times smaller than the atom. Ernest Rutherford & Nuclear Model of the Atom • Rutherford concluded that the results could be explained only if all the positive charge of the atom were concentrated in a tiny, massive central core. Rutherford’s model is therefore called the nuclear model of the atom. A Planetary Model of the Atom • The Bohr Model is probably familiar as the "planetary model" of the atom illustrated in the below figure that, for example, is used as a symbol for atomic energy. Atomic Spectra • The set of light wavelengths emitted by an atom is called the atom’s emission spectrum. • The emission spectrum of an atom can be seen by looking at the light through a prism or a diffraction grating. Spectroscopy is the study of spectra, that is, the dependence of physical quantities on frequency. Atomic Spectra • Emission spectrum of Hydrogen Emission Spectra of Hydrogen Atomic Spectra • Emission spectrum of Iron - Each line corresponds to a particular wavelength of light emitted by the atoms of the gas. Max Planck Quantum Theory • Awarded the Nobel prize in 1918 for his discovery of the quantized nature of energy. • The Energy of vibration of the atoms in a solid could only have specific frequencies as shown by: • E = nhf; where E = Energy; n is an integer such as 0,1,2,3…; h = 7 x 1034 J/Hz; f = frequency Excitation by absorption of light and de-excitation by emission of light Absorption Spectrum • A gas that is cool and does not emit light will absorb light at characteristic wavelengths. Periodic Table Emission/Absorption Spectra Applets • http://jersey.uoregon.edu/vlab/elements/El ements.html BOHR’S ATOM APPLETS • http://www.loncapa.org/~mmp/kap29/Bohr/app.htm • http://physics.gac.edu/~chuck/PRENHALL /Chapter%2031/AABXTEI0.html Incandescent Bodies • Incandescence is the release of thermal radiation from a body due to its temperature. • Molten glassy material glows orange with incandescence. The incandescent metal embers of the spark used to light this Bunsen burner emit light ranging in color from white to orange to red. This change correlates with their temperature as they cool in the air. • The temperature of lava flow can be estimated by observing its color. The color matches the measured temperatures of lava flows at about 1,000 to 1,200 °C. Radiation from Incandescent Bodies • As the temperature increases, the frequency at which the maximum energy increases. The spectral class of stars is equivalent to a classification of stars by their surface temperature, with higher temperatures to the left. What is a Blackbody? • An object is a "blackbody" if the radiation it emits into space originates completely from its temperature. Blackbody Radiation Applets • http://www.mhhe.com/physsci/astronomy/a pplets/Blackbody/frame.html • http://www.loncapa.org/~mmp/applist/blackbody/black.ht m Instructions for Blackbody Radiation Experiment • Using the applet (http://www.lon- capa.org/~mmp/applist/blackbody/black.ht m) find the wavelength(nm) and the temperature (K) for 25 different temperatures. What happens to the peak as the temperature is increased in the applet. Use an excel spreadsheet for your data. Program the spreadsheet to find the frequency for each wavelength. Use the Chart Wizard in excel to plot Temperature vs. Wavelength. Describe the graph. Write an equation for the graph and discover the constant (if any). • A student recognizes Einstein in a train and asks: Excuse me, professor, but does New York stop by this train? Relativity Review • http://www.phys.unsw.edu.au/einsteinlight • Twin paradox • http://www.phys.unsw.edu.au/einsteinlight/j w/module4_twin_paradox.htm Relativistic Paradoxes • This is a wheel, just an ordinary wheel, or is it? • Each successive image in the movie is rotated by a small amount compared to the previous image. • As the wheel rotates, the coordinates (x, y) of a point on the wheel relative to its centre change, but the distance r between the point and the centre remains constant • r2 = x2 + y2 = constant . Relativistic Paradoxes • What would happen if the wheel moved at speeds close to the speed of light? • We know that time slows down and lengths contract at relativistic speeds and mass increases…. Or does it? Relativistic Paradoxes • This is what a wheel looks like if the axle is moving at 87% of the speed of light. The cartwheel appears Lorentz contracted along the direction of motion. • The bottom of the cartwheel, where it touches the road, is not moving, and is not Lorentz contracted. You might think that the top of the cartwheel would have to move faster than the speed of light to overtake the axle moving at 87% of the speed of light; but of course it can't. • The cartwheel offers another example of the impossibility of completely rigid bodies in special relativity. In the frame of reference of someone riding on the axle (but not rotating), the rim is whizzing around and is Lorentz contracted, while the spokes that are moving transversely are not contracted. Something must give: the rim must stretch, or the spokes compress. • A Black Hole is a tunnel at the end of light. Relativistic Pizza Paradox • What would happen if the pizza moved at speeds close to the speed of light? Would someone be able to eat it? Would there be more pizza or less pizza? • We know that time slows down and lengths contract at relativistic speeds and mass increases…. Or does it? Solution • Does this mean you go faster than the speed of light? No. From the point of view of a person at rest on Earth, you never go faster than the speed of light. From your own point of view, distances along your direction of motion are Lorentz-contracted, so distances that are vast from Earth's point of view appear much shorter to you. Fast as the Universe rushes by, it never goes faster than the speed of light. Solution • It would take a huge amount of energy to keep you accelerating at g. Also, you would use up a huge amount of Earth time traveling around at relativistic speeds. If you took a trip to the edge of the Universe, then by the time you got back not only would all your friends and relations be dead, but the Earth would probably be gone, swallowed by the Sun in its red giant phase, the Sun would have exhausted its fuel and shriveled into a cold white dwarf star, and the Solar System, having orbited the Galaxy a thousand times, would be lost somewhere in its milky ways. credits • http://casa.colorado.edu/~ajsh/sr/contracti on.html • http://en.wikipedia.org/wiki/Special_relativit y • http://www.phys.unsw.edu.au/einsteinlight/ • http://www.thinkarete.com/quotes/by_teac her/albert_einstein/