Section 3_2 Notes
advertisement

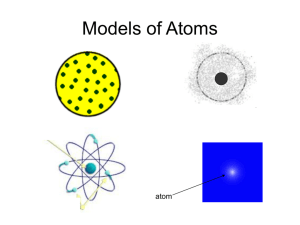
Section 3.2 Notes Chemistry Class Ms. Cox Key Notes The Structure of the Atom -The Atom: the smallest particle of an element that retains the chemical properties of that element. -The Nucleus: the small, dense region consisting of protons and neutrons at the center of an atom. The nucleus includes at least one positively charged proton and usually one or more neutral particles called neutrons. Hydrogen is an exception as it has only a proton and no neutron. -Electrons surround the nucleus in a much larger region. They are negatively charged particles. In a neutral atom, there is the same number of electrons as protons. -Cathode-Ray Tubes glow when electric current is passed through it. Scientists thought that a stream of particles called a cathode ray, traveled from the cathode to the anode and caused the glow. They found that these rays could be deflected by a magnet or away from a negatively charged object. This led to the hypothesis that particles in a cathode ray are negatively charged. Experiments carried out by English physicist Joseph John Thompson in 1897 supported this hypothesis. He was able to calculate charge to mass of these particles and found that the ratio was the same no matter the metals used for the cathode and anode. The ratio was also the same no matter was gas in the tube was used. SO…Thomson concluded the rays were composed of negatively charged particles called ELECTRONS. The atoms in the cathode-ray experiment were releasing electrons! This was evidence that atoms WERE divisible and that electrons were present in different kinds of atoms. - Electrons have a large charge to mass ratio. -In 1911, Ernest Rutherford conducted his now famous gold foil experiment. During the experiment, alpha particles bombarded a thin piece of gold foil. The positively charged alpha particles were expected to pass easily through the thin gold foil. Every now and then, however, an alpha particle bounced back- an unexpected result. Rutherford concluded that these particles were striking a tiny region of positive charge. -Every different atom possesses a different number of protons. So the number of protons determines and atom’s identity. AND for every proton in the nucleus of an atom, there is the SAME number of electrons surrounding the nucleus. -Forces in the nucleus: Generally, particles that have the same electric charge repel one another. Nuclear forces keep protons and neutrons together in a nucleus.