Chemistry 331: Organic Chemistry Fall 2014, University of Delaware Prof. Donald Watson
advertisement

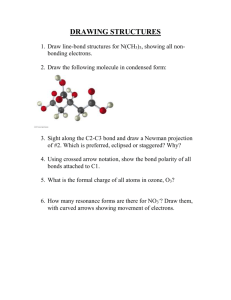
Chemistry 331: Organic Chemistry Fall 2014, University of Delaware Prof. Donald Watson Problem Set 2 Due Beginning of Class 9/25/14 You may work in groups. Please write answers in the space below, for problems form the book, please attached them on separate sheet(s) of paper. Each student must turn in their own, handwritten answer set to get credit. Please write answers on this page. 1. Draw the additional resonance structures for the following: 2. Consider 1-bromo-2-methylpropane and draw the following: (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energy 3. Draw the Newman projections of 2,2,4-Trimethylpentane looking down the C3-C4 bond. Label the most stable confirmation. 4. Predict the products for the following reaction: 5. Draw the arrow-pushing mechanism for the following reaction. Draw and label the Markovnikov and anti-Markovnikov products. Which one is the more likely product of the reaction? Explain. HBr 6. Assign E or Z configurations to these alkenes: 7. Label all the double-bonds in retinal, an essential molecule in our eyes that allows us to see, as cis or trans. Me Me Me Me retinal O 8. Determine the degree of unsaturation for the following compounds: 9. Draw the two isomers of cyclooctene (cis and trans). Which is more stable and why? 10. One of the following molecules is a stable organic molecule while the other is not. Using your knowledge of molecular orbitals and atomic geometry, predict which is unstable and explain why. (Hint: build a model!). 11. How many signals will appear in the 13C NMR of the following compounds: H 3C CH 3 CH 3 CH 3 The following problems are highly recommended, but not required for this problem set: 2.42-2.53, 2.55, 2.57,2.58, 2.65, 2.67, 2.69-2.71,