Naming Cycloalkane Compounds KEY - SASC Specialists
advertisement

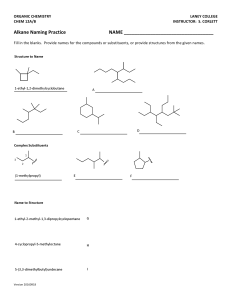
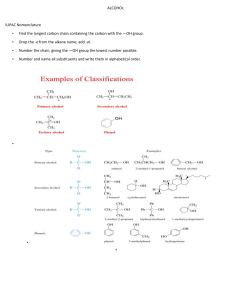
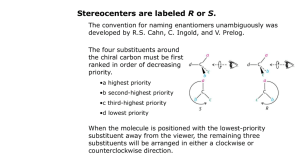
Jim Hollister/Rolf Unterleitner SASC/UC Davis Naming Cycloalkanes: Nomenclature Examples KEY Name the following molecules. 1) HO F NO2 Name: 4-fluoro-3-nitrocyclohexanol. (Since the alcohol has highest precedence, its carbon is given the lowest number. Precedence of groups are listed on nomenclature handout on workshop website.) 2) HO F CH2CH3 Name: 3-ethyl-4-fluorocyclohexanol 3) Cl Name: 1-(3-chloro-4-ethylcyclopentyl)-5-methylhexane, not 1-chloro-2-ethyl-4-(5methylhexyl)-cyclopentane 4) Name: 3-ethyl-1,1-dimethylcyclohexane Uses lowest numbers: 1,1,3 is lower than 1,3,3. (The second substituent, one of the methyls!, would have the lower number of 1 in the correct answer; in the incorrect answer, 1-ethyl-3,3-dimethylcyclohexane, the second substituents (the methyls) would each have a 3.) 5) Name: 1-ethyl-3-methylcyclohexane Uses alphabetical rule to determine C-1. 6) Cl Name: 1-chloro-2-(2-methylpentyl)-cyclopentane When there is a tie in the number of carbons between a cyclic alkane and a regular alkane, choose the cycloalkane as the parent chain. 7) Name: cis-1-(1-methylethyl)-3-propylcycloheptane or cis -1-isopropyl-3-propylcycloheptane 8) Br Name: 4-bromo-1-ethyl-2-methylcyclopentane Warning: Stereochemical information on the substituents is not indicated in the answer. This requires knowledge from chapter 5. ( You can't use cis and trans because there are three groups on the ring; R and S would be used.) 9) F Name: trans-4-(2,2-dimethylpropyl)-3-fluoro-1,1-dimethylcycloheptane or trans-3-fluoro-1,1-dimethyl-4-neopentylcycloheptane Even though there are more than two substituents on the ring, you can use trans because the methyl groups are on the same carbon and they do not confuse the meaning of using cis or trans.