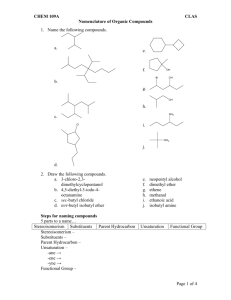
Compounds with two double bonds are named as a diene with two
advertisement

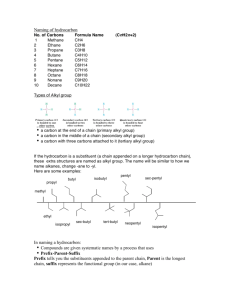
Organic chemistry and Biological chemistry for Health Sciences 59-191 Lecture 7 Hydrocarbons of all types have some common physical properties: Have little if any overall polarity Insoluble in water but dissolves in non-polar solvents Generally less dense than water Like dissolves like rule: Whether a solvent can dissolve some substance depends on the likeness in polarity between the solute and the solvent. Polar solvent dissolve polar and ionic compounds and nonpolar solvents dissolve nonpolar compounds Polar solvents such as water are good for dissolving polar and ionic compounds, like sugar or salt. Polar molecules and ions can attract water molecules around themselves, form solvent cages, and in this form freely intermingle with water molecules. Nonpolar solvents, like gasoline, do not dissolve sugar or salt, because nonpolar solvent mole-cules cannot be attracted to polar molecules and ions and form solvent cages. Naming of alkanes and cycloalkanes: All organic compounds can be named by following a set of rules. The formal one is known as IUPAC rules, after the international Union of Pure and Applied chemistry. All chemical societies in the world accept the IUPAC. IUPAC RULES FOR NAMING THE ALKANES: The name ending for all alkanes (and cycloalkanes) is –ane. The parent chain is the longest continuous chain of carbons in the structure. A prefix is attached to the name ending –ane, that specifies the number of carbon atoms in the parent chain. Meth-1C But- 4C Hept-7C Eth- 2C Pent-5C Oct-8C Prop-3C Hex-6C Non-9C Dec-10C The carbon atoms of the parent chain are numbered starting from whichever end of the chain gives the location of the first branch the lower of two possible numbers. Name each branch attached to the parent chain Alkyl groups: Any branch that consist of carbon and hydrogen and has only single bonds is called an alkyl group, and the name of the alkyl groups end in -yl. (alkyl group-an alkane minus one H) Primary carbon: Carbon is directly attached to just another carbon Secondary carbon: Carbon is directly attached to two other carbons Tertiary carbon: Carbon is directly attached to three other carbons Attach the name of the alkyl group to the name of the parent as a prefix. Place the location number of the group in front of the resulting name and separate the number from the name by a hyphen When two or more groups are attached to the parent, name each and locate each with a number. The names of alkyl substituents are assembled in alphabetical order (always use hyphens to separate numbers from words). When two or more substituents are identical, use such prefixes as di(for 2), tri (for 3)and so forth and specify the location number of each group. Always separate a number from another number in a name by a comma. IUPAC rules for Naming Cyclohexane: To name a cycloalkane, place the prefix cyclo- before the name of the straight chain alkane that has the same number of carbon atoms as there are in the ring. When necessary, give numbers to the ring atoms by giving location 1 to a ring position that holds a substituent and numbering around the ring in whichever direction reaches the nearest substituent first When halogen atoms, or nitro groups are joined to a carbon or ring the following name is used -F Fluro -NO2 Nitro -NH2 Amino The prefix iso- in the name of an alkyl group, such as isopropyl or isobutyl, has a special meaning. It can be used to name an alkyl group that has the following feature: A word fragment is attached to iso which specify the total number of carbons in the alkyl group. Isopropyl has three carbons (indicated be prop), Isobutyl has four carbons (indicated by but) and so on. NAMING THE ALKENES: The following guideline is used to name the alkenes: Use ending –ene for all alkenes and Cycloalkenes For open chain alkenes, identify the parent chain as the longest sequence of carbons that includes the double bond. For cyclic alkenes, the ring is the parent in all situations we will encounter. For open-chain alkenes, number the parent chain from whichever end gives the lower number to the first carbon of the double bond. This rule gives precedence to the location of double bond over the location of the substituent on the parent chain For cycloalkenes, always give position 1 to one of the two carbons at the double bond. To decide which carbon gets this number, number the ring atoms from carbon 1 through the double bond in whichever direction reaches a substituent first. To the name begun with rules 1 and 2, place the number that locates the first carbon of the double bond as a prefix, and separate this number from the name by a hyphen. If substituents are on the parent chain or ring, complete the name obtained by rule 5 by placing the names and location numbers of the substituents as prefixes. Separate number from numbers by commas, but use hyphens to connect number to a word. Compounds with two double bonds are named as a diene with two numbers in the name to specify the locations of the double bonds. This pattern can be easily extended to trienes, tetraenes and so on.