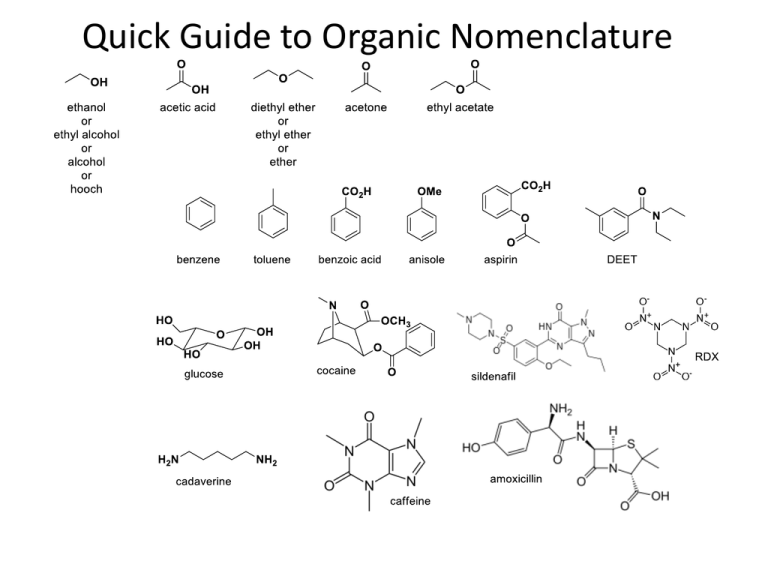
Quick Guide to Organic Nomenclature
advertisement
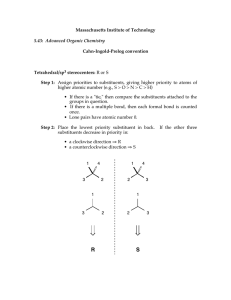
Quick Guide to Organic Nomenclature Pick longest carbon chain as base, assign numbers so that they are as low as possible Are two compounds the same? Can they be “superimposed”? Yes same compound No different compounds Are two compounds the same? Neither of these can be superimposed with any of the others Therefore, each is different from one another and from the other two. Flip over - same structure Therefore, these are the same compound All have the same molecular formula (C11H24O). Therefore, the different compounds are structural isomers. More complex alkanes 1. Pick longest carbon chain as base (or, perhaps, cycloalkane or aromatic). 2. Determine substituents; assign numbers so that you have the lowest set of numbers. 3. Name is list of substituents in alphabetical order, preceeded by number. More complex alkanes 1. Pick longest carbon chain as base (or, perhaps, cycloalkane or aromatic). 2. Determine substituents; assign numbers so that you have the lowest set of numbers. 3. Name is list of substituents in alphabetical order, preceeded by number. Longest carbon chain = 8 : thus, it is an octane Substituents: methyl, chloro, fluoro Either 2, 5, 6 or 3, 4, 7 – go with 2, 5, 6 5-chloro-6-fluoro-2-methyloctane All numbers and letters separated by dash. Chain = 9: nonane Base = nonylamine (NH2 – amine) Substituents: 2 methyls 3,5-dimethyl-5-nonylamine Chain = not applicable Base = cyclohexanol (6 membered ring of carbons) Substituents: bromo, amino, isopropyl 3-amino-2-bromo-5-isopropylcyclohexanol Chain = 12: dodecane Alkene dodecene Base = dodecenol (alcohol) Substituents: chloro, phenyl (benzene ring), n-propyl 4-chloro-7-phenyl-6-n-propyldodec-3-en-2-ol