Nomenclature Flow Chart
advertisement

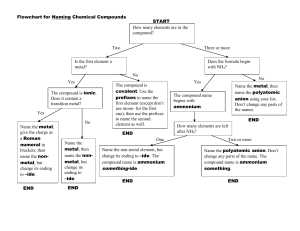
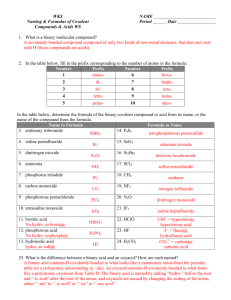
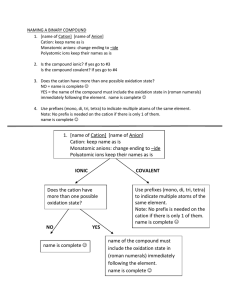
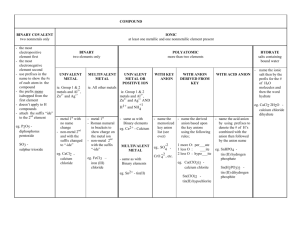
Chemical Nomenclature and the naming of compounds Chemical Nomenclature Map YES Ionic Compound Follow Case 1 for Type 1 metals OR Case 2 for Type 2 metals Does the compound start with a metal or NH4? YES Acid Follow Case 3 NO Does it start with H? NO Covalent Compound Follow Case 4 Case 1: First atom is a Metal and has only one possible charge. (type 1 metal) (NH4 also fits here) Use Periodic Table of Ions. No prefixes are used! The Name of Compound = name of cation + name of anion (nonmetal w/ide suffix or name of polyatomic ion). Examples: NaF = sodium fluoride; Na3PO4 = sodium phosphate; (NH4)3PO4 = ammonium phosphate Writing Formula: Look up cation (metal ion) and anion (nonmetal ion) charges must cancel. Reduce and crisscross if charges don’t cancel. Case 2: First atom is a Metal BUT can have more than one charge. (type 2 metal) Use Periodic Table of Ions. No prefixes are used! The Name of the Compound = name of cation + the charge of cation using roman numerals in parenthesis + name of anion Examples: PbCl2 = Lead (II) chloride; Cu(NO3)2 = copper (II) nitrate Case 3: First atom is a “H”. These are acids and are a unique ionic compound. All Acidic compounds end in the name “Acid” make sure to add it to all names. Look up the anion ion the Periodic Table of ions. (several options below, read carefully) o If anion ends in –ide, add the prefix hydro- to the root name and change –ide to –ic. [HCl = hydrochloric acid] o If anion ends in –ate, write the root name and change –ate to –ic. [HClO3 = chloric acid] o If anion ends in –ite, write the root name and change –ite to –ous. [HClO2 = chlorous acid] Writing formulas: If the name ends in the word acid, then the formula starts with a hydrogen ion (H+) o Anion depends on root word and ending root-ic root-ate root-ous root-ite hydro- root-ic root-ide Case 4: First atom is a Non-Metal or are covalent compounds with two nonmetals only. Use prefixes to indicate the number of atoms. The mono prefix is not used with the first element. o (mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, deca-) The Name of Compound = prefix of the first element + name of first element + prefix of the second element + name of second element with -ide suffix. Examples: CO = carbon monoxide; NO2 = nitrogen dioxide; N2O = dinitrogen monoxide; P2O5 = diphosphorus pentoxide