Lewis Diagram, naming flow charts
advertisement

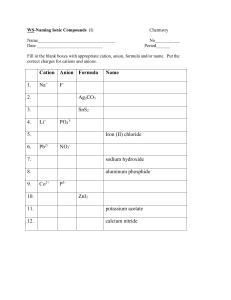
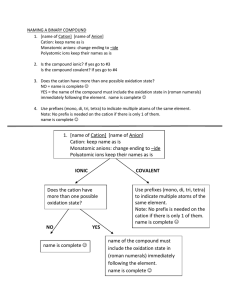
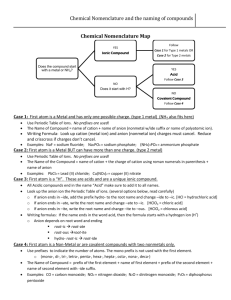
Draw the Lewis Diagram for KNO2 1 Draw the Lewis Diagram for NaNO3 2 etal onm on l + N i Meta yatomic l o p r o in , on I, II Ag i t Ca oup Cd, Gr , Zn Al Ionic Single Charged Cation • Name the cation • Change the ending of the anion name to "­ide" • Never change the name of a polyatomic ion Compound Non met al + Non met al An y cat othe ion r m Molecular eta l Multiple charged Cation • Name the cation • Put cation charge in () using roman numerals after cation name • Change the ending of the anion name to "ide" • Never change the name of a polyatomic ion • Use prefixes in front of element name to signify quantity in molecule • Do not use mono on first element, use it everywhere else • Change last element in compound to end in "­ide" 3 etal onm on l + N i Meta yatomic l o p r o Ionic • Determine the cation and anion • Alternate cation and anion in [ ] • Put the charge outside of each • Cation will have lost electrons, Compound Non met al + No nme tal Molecular • Count all valence electrons • Add or subtract from total if the molecule is charged • Put least electronegative element in center • Single bond elements together from center out • Complete octets on outer elements (any leftovers will go on center element) • Anion will have gained electrons 4 5