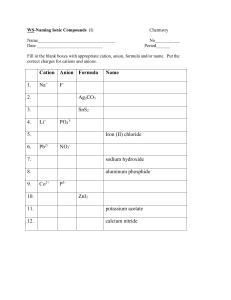
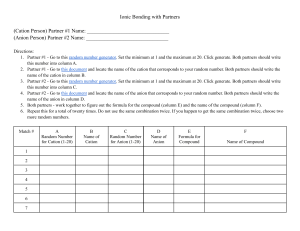
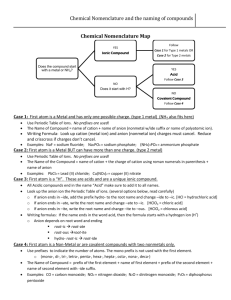
Naming Binary Compounds & Acids: A Chemistry Guide
advertisement
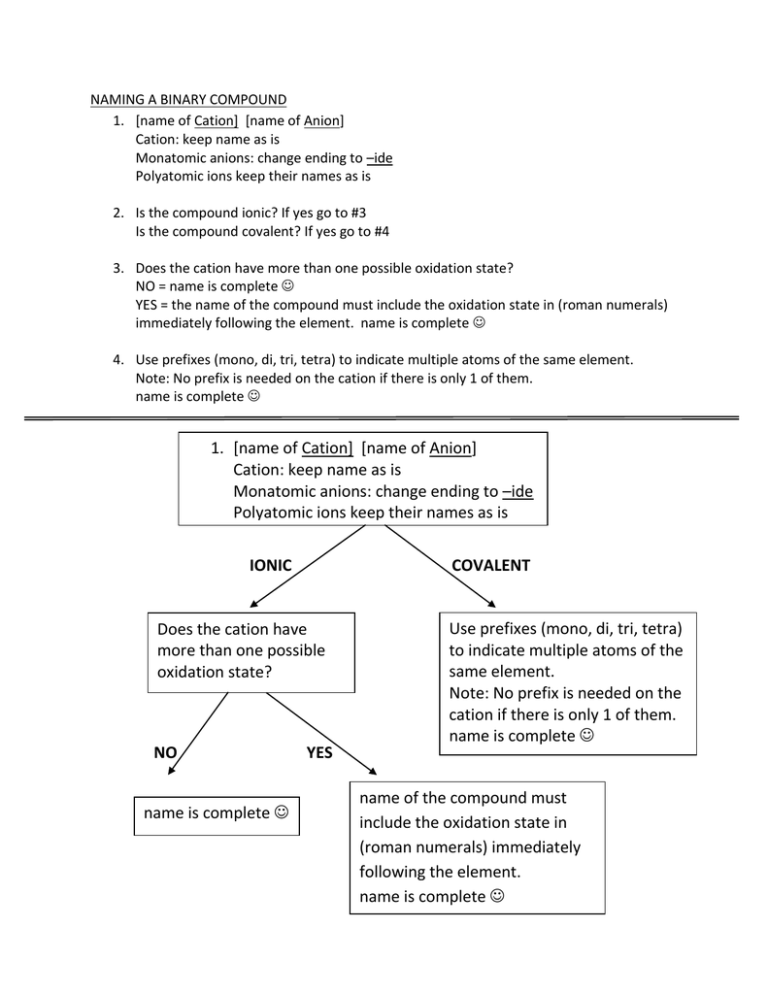
NAMING A BINARY COMPOUND 1. [name of Cation] [name of Anion] Cation: keep name as is Monatomic anions: change ending to –ide Polyatomic ions keep their names as is 2. Is the compound ionic? If yes go to #3 Is the compound covalent? If yes go to #4 3. Does the cation have more than one possible oxidation state? NO = name is complete YES = the name of the compound must include the oxidation state in (roman numerals) immediately following the element. name is complete 4. Use prefixes (mono, di, tri, tetra) to indicate multiple atoms of the same element. Note: No prefix is needed on the cation if there is only 1 of them. name is complete 1. [name of Cation] [name of Anion] Cation: keep name as is Monatomic anions: change ending to –ide Polyatomic ions keep their names as is IONIC COVALENT Does the cation have more than one possible oxidation state? NO name is complete YES Use prefixes (mono, di, tri, tetra) to indicate multiple atoms of the same element. Note: No prefix is needed on the cation if there is only 1 of them. name is complete name of the compound must include the oxidation state in (roman numerals) immediately following the element. name is complete OXYANIONS NAMING ACIDS • If it has hydro- in the name it has no oxygen (Anion ends in –ide) • • No hydro in name, anion ends in -ate or –ite Write anion and add enough H to balance the charges.