Naming Ionic Compounds and Covalent Compounds
advertisement

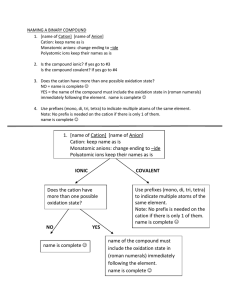
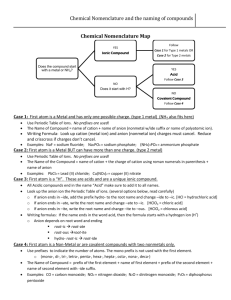
Do Now: • Creative Assignment – You are a CATION – You are writing to an ANION • Write a short Love Note about the topic: “Let’s bond together” Email to ddavis@scischina.org You have 5 minutes!!!!! HAVE YOUR HW OUT ON YOUR DESK! Trade Papers and Check Check Answers Steps to NAMING ionic compound: 1. Name the cation 2. Name the anion and change the ending to “ide” - Group 17: ine ide Oxide Sulfide Selenide Phosphide Silicide nitride hydride 3. Add (roman numeral) if the cation is a transition metal Steps: 1. Name the cation 2. Name the anion and change the ending to “ide” - Group 17: ine ide Oxide Sulfide Selenide Phosphide 3. Add (roman numeral) if transition metal 1. Calcium and Bromine Naming Ionic Compounds Examples: 2. MgS Try #1-4 on Ionic Formulas HW Steps: 1. Name the cation 2. Name the anion and change the ending to “ide” - Group 17: ine ide Oxide Sulfide Selenide Phosphide 3. Add (roman numeral) if transition metal Naming Ionic Compounds w/ Transitional Metals Examples: **Work Backwards** 1. FeO 2. CrBr3 Name the following in your notebook: 1. Pb3N2 Then TRY #12-14 from formula HW 2. CdBr2 3. NiI3 Steps to NAME an ionic compound with Polyatomic Ions: 1. Name cation (or ammonium only “+” p.i.) 2. Name polyatomic ion *** DO NOT CHANGE ANYTHING*** Example: Mg(NO3)2 Molecular / Covalent Compounds • A compound that is composed of 2 or more non-metals • How to recognize an molecular/covalent compound … • Anion and anion • Nonmetal and nonmetal • and - Steps to NAMING a covalent compound : 1. Name the first element in formula 2. Name second element in the formula and change endings to “ide” 3. Add prefixes before each element name (according to the subscripts) 4. EXCEPTION never use mono for the 1st element Prefixes to MEMORIZE Number 1 2 3 4 5 6 7 8 9 10 Prefix Steps: 1. Name first element in formula 2. Name second element and change endings to “ide” 3. Add prefixes (according to how many there are) 4. EXCEPTION never use mono for the 1st element 1. CO2 Naming Covalent Compounds Examples: 2.N3F8 Try #9-11on Naming Covalent Compounds Worksheet Steps to Write the Formula for a covalent compound : 1. Write the symbol for the first element in the compound. 2. Write the formula for the second element in the formula. 3. Add the prefixes as subscripts for each element. • • NO REDUCING NO CHARGES Ex 1: Carbon Monoxide Ex 2: Diphosphorus tetrachloride HW