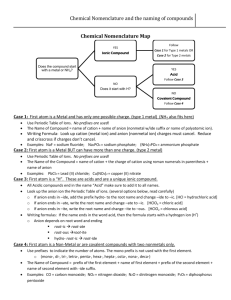
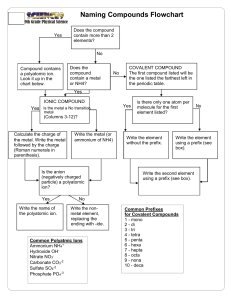
Flowchart for Naming Chemical Compounds START How many elements are in the compound? Two Three or more Is the first element a metal? Does the formula begin with NH4? No Yes The compound is ionic. Does it contain a transition metal? Yes Name the metal, give the charge as a Roman numeral in brackets, then name the nonmetal, but change its ending to –ide. END No The compound is covalent. Use the prefixes to name the first element (except don’t use mono- for the first one), then use the prefixes to name the second element as well. END Name the metal, then name the nonmetal, but change its ending to –ide. END One Name the non-metal element, but change its ending to –ide. The compound name is ammonium something-ide. END No Yes Name the metal, then name the polyatomic anion using your list. Don’t change any parts of the names. The compound name begins with ammonium. END How many elements are left after NH4? Two or more Name the polyatomic anion. Don’t change any parts of the name. The compound name is ammonium something. END Flowchart for Writing Formulas for Chemical Compounds START Does the name contain prefixes? Yes No Is the first name ammonium? The compound is covalent. Write the symbol for each element and use the prefixes to find the subscripts. END No Yes The compound is ionic. Does the name contain a Roman numeral? Yes The compound is ionic and contains NH41+. Is the anion name on the list of polyatomic anions? No The Roman numeral gives the charge on the metal ion. Use this when balancing the charges. Determine the charge of the cation from the periodic table (the group number). Is the anion name on the list of polyatomic ions? No Yes Yes No Find the charge of the anion using the periodic table and use this with the cation charge to balance the charges. You can draw Lewis dot (electron transfer) diagrams if you like. Use the charge of the polyatomic anion with the charge of the cation to balance the charges. Write the formula using subscripts to show how many of each ion are needed to balance the charges. END