Name
advertisement

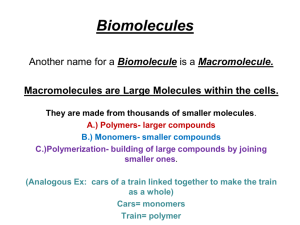
Biochemistry in Biology 1. Organic molecules are generally considered as molecules that contain the element carbon. (Although there is an exception to the rule- CO2 ) Inorganic substances are those substances without carbon. 2. When there is only ONE straight line between atoms it is called a single bond. 3. When there are TWO straight lines between atoms it is called a double bond. 4. There are 4 major groups of organic molecules a. carbohydrates b. lipids c. proteins d. nucleic acids Carbohydrates All carbohydrates are made of a long string of sugars. The major function of carbohydrates in the body is for “quick energy.” All sugars are made of carbon, hydrogen, and oxygen. Examples of carbohydrates would include sugars and starches. There are 3 sizes of carbohydrates depending on the amount of sugar included in the carbohydrate. 1. Monosaccharides are made of one sugar a. Examples: glucose, galactose, & fructose b. 2:1 ratio of hydrogen and oxygen c. Building blocks of monosaccharides are sugars d. All end in -OSE "D" stands for dextro and "L" stands for levo. If the OH is on the right, then we are dealing with a "D" sugar, in this case D-glucose. Each of these sugars is a "D" sugar because the OH on the last asymmetric carbon atom is written on the right ..on PPT Glucose (blood sugar) Fructose (fruit sugar) Galactose (broken down milk sugar) Write the molecular formulas for the 3 sugars drawn above. (count each element) Glucose: C6H12O6 Fructose: C6H12O6 Galactose: C6H12O6 e. 2. Disaccharides are made of double sugars and sucrose, maltose, and lactose are three examples. 3. Polysaccharides are made of many monosaccharide sugars linked together. a. Also called starches b. Three types of polysaccharides: i. Starch ii. Glycogen- animals iii. Cellulose- plants Dehydration Synthesis When you want to make a Disaccharide, you must remove a hydroxal group (OH) from one monosaccharide and a hydrogen (H) from the other monosaccharide. So, we have 2 hydrogens and 1 oxygen, which we can turn into water (H2O). This process is called dehydration synthesis. Synthesis means “to to make_” and dehydration is the removal of water. So, we make disaccharides by removing a water molecule from the union of 2 monosaccharides. Hydrolysis In order to break down starches, you must use water to break the bond and re-establish the 2 monosaccharides. We add an OH group to one and a H to the other. So, in theory, if you eat starches and do not drink water you have no way of getting the energy out of the starch. If the starch isn’t used immediately, it will turn to fat. Lipids Lipids have a large energy potential, but a slow release. To better understand the chemistry of fats, it is helpful to study first the small molecules which join to make up fats. Fat molecules are made up of 2 small “building blocks” called glycerol and fatty acids. Glycerol 1. 2. 3. 4. 5. Caproic Acid (fatty acid example) What elements are present in glycerol? Carbon, hydrogen, & oxygen What is the molecular formula for glycerol? C3H8O3 What elements are present in all fatty acids? Carbon, hydrogen, & oxygen What is the molecular formula for caproic acid? C6H12O2 Examples of Lipids: a. fats b. oils c. waxes Proteins Proteins are complex molecules made up of smaller “building blocks” called amino acids. The element nitrogen (N) is found in all amino acids along with C, H,O. Proteins can be found in places like muscles for contractions, transporting oxygen, in the blood, providing immunity to diseases, and carrying out chemical reactions. Alanine 1. 2. 3. 4. Valine What is the molecular formula for alanine? C3H7O2N What is the molecular formula for valine? C5H11O2N Draw a triangle around each amino group (NH2). Do carbohydrates have amino groups? No Nucleic Acids A nucleic acid is a complex macromolecule that stores information in cells in the form of a code. Nucleic acids have building blocks called nucleotides. They are made of C, H, O, N, and P atoms. As a result, they are in control of what you look like and everything genetic about you. These pieces of information are passed from one generation to the next. 2 Nucleic Acid Examples: 1. Deoxyribonucleic acid (DNA) 2. Ribonucleic acid (RNA) Structure of a nucleotide: Double Helix of DNA: