Workshop Exercise #9
advertisement

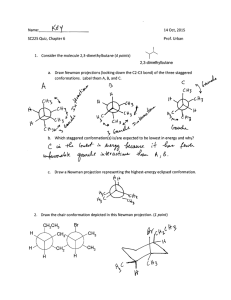
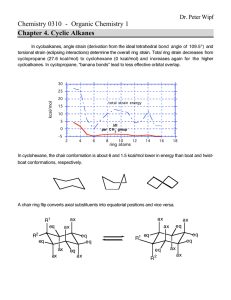
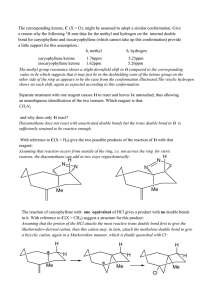
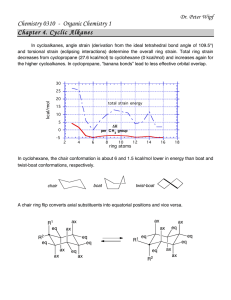
Chemistry 255 Prof. Loyd Bastin Workshop Exercise #9 (11/4/08) Fall 2008 1. Although a chlorine atom is about the same size as a methyl group, the conformation of 1,2-dichloroethane in which the two chlorines are eclipsed is higher in energy than the conformation of butane in which the two methyl groups are eclipsed. Explain. Be sure to draw Newman projections. Hint: Size isn’t everything! 2. Answer the following questions concerning cis-1,3-dimethylcyclohexane a) Draw the two chair conformations and label both methyl groups as axial or equatorial. b) Label the higher energy conformation and the lower energy conformation. c) The energy difference in these two conformations has been measured to be about 5.4 kcal/mol. How much of this energy difference is due to the torsional energy of gauche relationships between the methyl groups and the ring? Remember that each gauche interaction is worth about 0.9 kcal/mol. d) How much energy is due to the additional steric strain of the 1,3-diaxial interaction? 3. Normally, a trans alkene is more stable than its cis isomer. Trans-Cyclooctene, however, is less stable than cis-cyclooectene by 9.2 kcal/mol. Explain.