Chap 3 Solns-5/E
advertisement
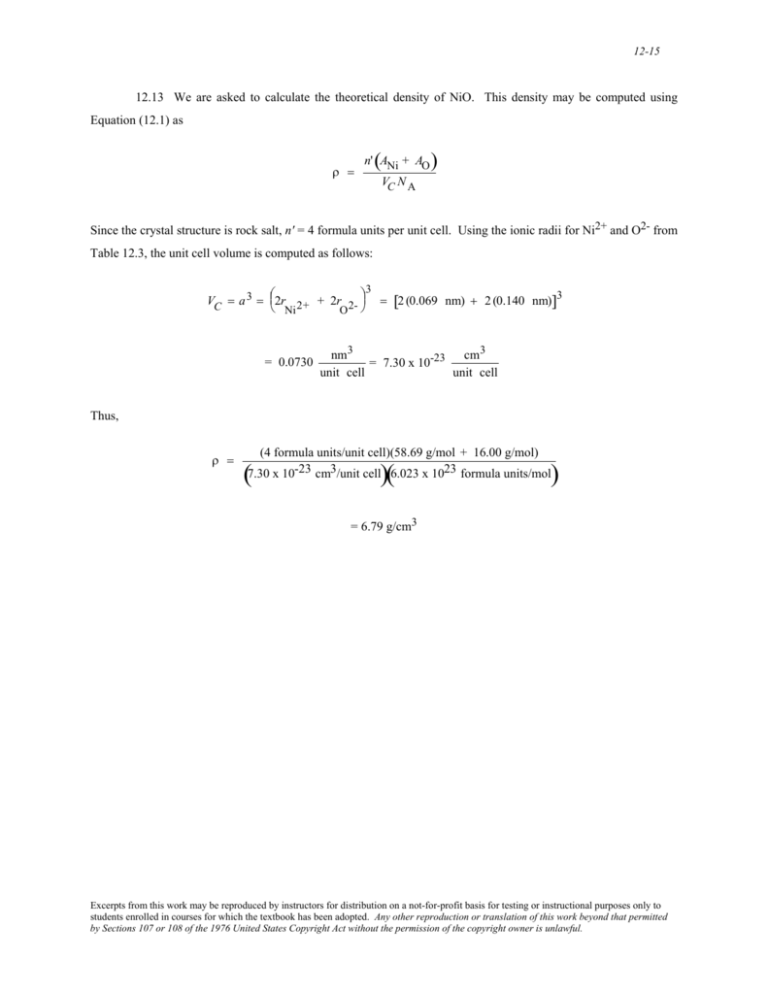
12-15 12.13 We are asked to calculate the theoretical density of NiO. This density may be computed using Equation (12.1) as ρ = ( n' ANi + AO ) VC N A Since the crystal structure is rock salt, n' = 4 formula units per unit cell. Using the ionic radii for Ni2+ and O2- from Table 12.3, the unit cell volume is computed as follows: ⎛ ⎞3 VC = a 3 = ⎜2r 2+ + 2r 2- ⎟ = [2 (0.069 nm) + 2 (0.140 nm)]3 ⎝ Ni O ⎠ = 0.0730 cm 3 nm3 = 7.30 x 10-23 unit cell unit cell Thus, ρ = (4 formula units/unit cell)(58.69 g/mol + 16.00 g/mol) 7.30 x 10-23 cm3/unit cell 6.023 x 1023 formula units/mol ( )( ) = 6.79 g/cm3 Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for testing or instructional purposes only to students enrolled in courses for which the textbook has been adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.











