Revised may 2013 Electro Chemistry notes (chapter 20 of Glencoe
advertisement

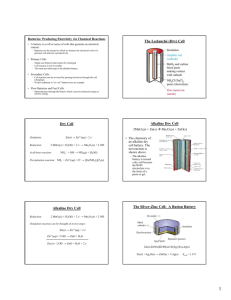
Revised may 2013 Electro Chemistry notes (chapter 20 of Glencoe textbook) There are two basic types of electrochemical cells: Voltaic or Galvanic or commonly called a cell (or battery) best pictures in your book on pages 709, 736 : These cells spontaneously produce a direct current (flow of electrons) Electrolytic cell or plating reaction: best pictures page 732 and 737. This cell uses energy (from a battery or D.C. (direct current) source) to reverse natural reactions. We put expensive silver or gold on top of cheaper iron or zinc. We can split water back into hydrogen and oxygen. Separate NaCl into Chlorine gas and sodium. Etc. Note: a battery is a collection of cells: you can have a single “D” cell or a collection of “D” cells called a battery. A “9 volt battery” is really six(6) 1.5 volt cells in single package). Using RedOx terminology to understand electrochemistry: Oxidation occurs at the Anode: oxidation is loss of electrons, electrons are negative. The anode is sacrificed (lost as soluble ions) Reduction occurs at the Cathode: reduction is gain of electrons. One terminal of a Cell (battery) is the anode one terminal is the cathode. Which we call a negative terminal should be easy (what does anode sound like) A Salt bridge separates the half cells. Reduction occurs in one half cell and Oxidation occurs in the other half cell. The salt (or electrolyte) just maintains electrical neutrality in a complete cell. Some people categorize cells (batteries) into two types: Disposable and rechargeable. Some cells (batteries) are designed to be used up then thrown away. They come in a variety of sizes and voltages: D, C, AA, AAA (and AAAA) are the most common; all are about 1.5 volts. There are two basic formulas the “heavy duty” (older style with an acidic MnO2 paste, zinc and a carbon cathode) and Alkaline as described on page 667-668) Rechargeable cells can have energy pumped back into them to reverse the natural chemical reaction and “Store” this energy. They are of a different physical design and different materials. The anode is able to reform in a predictable manner (or at least semi-predictable). Eventually the physical structure is broken down and the Reusable cell fails. The first dependable “wet” cell was the Daniel cell using a Zinc anode and a Copper cathode. See page 674 The standard reduction (or oxidation) potentials are for a 1 molar solution of ions at 25oC. The potential (voltage) numbers would be slightly different at different conditions. (cold batteries don’t work quite as well as room temp batteries) Lithium is easily oxidized (therefore not easily reduced). The value of zero (0.0 volts) is assigned to Hydrogen. LOOK UP the different elements (species) and see what would oxidize and what would reduce (each does what it is better at). If you must turn the equation around (change an oxidation into a reduction or visa-versa) the value of the number is opposite (-1.66v becomes +1.66 volts) Battery shorthand and cell potentials name: ________________ period: ___ Electrical potential = 1.10 V for 1.0 M solutions at 25oC What type of ½ reaction is occurring at this anode? Oxidation or reduction Looking at the table of standard reduction potentials (on back or in book) you can determine the potential for each reaction (for the oxidation reaction that occurs at the anode you will need to reverse the equation so you must reverse the potential as well) Zn0 Zn+2 + 2e- 0.76 V (the opposite of the reduction potential) Cu+2 + 2e- Cu0 0.34 V The sum of these reactions would have a potential of 0.76 + 0.34 or 1.10 volts; a standard Daniel cell potential is 1.10 volts (when operating at 25 oC with solutions of 1.0 Molar concentration) This battery can be written in short hand as: Zn | Zn+2 | | Cu+2 | Cu Read as zinc is oxidized at the anode to zinc +2 cations and copper +2 cations are reduced to copper metal Practice using the shorthand notation and the reduction potential chart to determine the voltage of several possible electrochemical cells Suggestions: Au and Fe; Mg and Zn; Li and H2; Sn and Ag; Fe and Cu