notes
advertisement

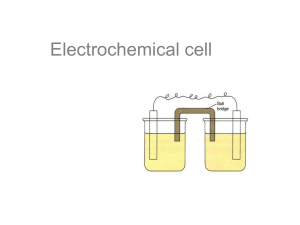
Unit 5: Electrochemistry The oxidation number of an atom in a molecule is defined as the electric charge an atom has. Balancing Redox Reactions: An example with acid. First, identify what is being oxidized and reduced. Find the two half reactions. Then, balance the half reactions for mass. Now, balance the half reactions for charge. We then take the half reactions and multiply them by the appropriate factors to make the number of electrons on each side equal: Add the two balanced half reactions: Eliminate commons reactants and products: Electrochemical cells are very closely related to oxidation-reduction reactions because the transfer of electrons results in a potential difference. Electrons flow through the wire from the Zn electrode (anode) to the Cu electrode (cathode). Electrons always flow from the anode to the cathode. –(mnemonic: alphabetical order) Oxidation takes place at the anode, reduction at the cathode. –AnOx, RedCat Ions flow through the salt bridge in the opposite direction of the electrons. All About Potential The standard potential Eo is a quantitative measure of the tendency of the reactants in their standard states to proceed to products in their standard states. Free energy is associated with the same characteristics, and is defined as: – ∆Gorxn= -nFEo where n=number of moles of electrons transferred in a balanced redox reaction and f is the faraday constant, 9.65x104 Calculating Cell Potential All potentials listed are for reduction reactions; the sign must be switched for oxidation. The more positive the value of the reduction potential, the reaction as written is more likely to occur as a reduction. Given two half reactions, the one with the more positive Eo is the one that will occur as on oxidation. Changing the stoichiometric coefficients for a half-reaction does not change the value of Eo. Non-standard conditions E=Eo – (RT/nF)ln(Q) –Q= Reaction quotient Q=[products]/[reactants] –F= Faraday constant 9.65x104 joules/(volts*mole) –R= Gas constant Mass Current Current I (amperes, A) = –charge (coulombs, C) / time (seconds, s) Example: –A current of 1.50 A is passed through a solution containing silver ions for 15.0 minutes. The voltage is such that silver is deposited at the cathode. What mass of silver is deposited? Ag+(aq) + e- Ag(s –Calculate the charge passed in 15.0 minutes: Q=I*t=(1.5A)(15.0 min)(60.0 sec/min)=1350 C –Next, calculate the number of moles of electrons: (1350 C) ((1 mol e-)/(9.65x104 C))=0.0140 mols e–Finally, calculate mass of silver deposited: (0.0140 mols e ) ((1 mol Ag)/(1 mol e )) ((107.9 g Ag)/(1 mol Ag))= 1.51 g Ag by the non-Italian Stallion Peter Bartoszek and the ever….present Jason Crystal