Redox Reveiw
advertisement

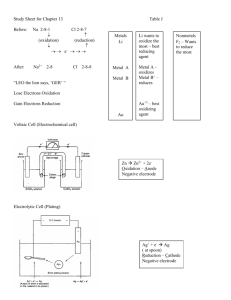
Redox Review LEO – Loss of electrons oxidation GER – Gain of electron reduction O↑L – Oxidation – Increase in oxidation # Loss of electrons R↓G – Reduction – Decrease in oxidation# Gain of electrons Use rules for assigning oxidation numbers, if no rule for an element figure it out using total of all oxidation numbers equals zero. Half reactions – Oxidation electron on right Reduction electrons on left Half reactions charges must balance on both sides Electrons are transferred from metal being oxidized to ion being reduced. If any oxidation number changes from one side of equation to the other it must be a REDOX reaction. (Look for one alone on one side and in compound on the other.) Spontaneous reactions occur when element above ion on Table J Total electrons transferred multiply coefficient by electrons or gained. Balance reaction by crisscrossing charge as coefficient. Electrochemical cells (voltaic) spontaneously use chemical reaction to produce electricity. Salt bridge transfers ions from one half cell to the other. RED CAT and AN OX - Anode is where oxidation occurs Cathode is where reduction occurs in any type of cell. Anode losses mass as metal undergoes oxidation (goes from element to ion) as the number of metal atoms decrease Cathode gains mass as ions undergo reduction (goes from ion to metal) and the number of metal atoms increase. Electrochemical cell anode is negative cathode is positive. Electrons flow from anode to cathode (higher to lower in table J) Electrolytic cell is nonspontaneous (requires power source) and anode is positive and cathode is negative. Electric energy turns to chemical energy. Elements above H2 on table J will react in acid because they are more active. Elements below H2 (Cu, Ag, and Au) on table J will not react in acid because they are less active than hydrogen.