ELECTROCHEMICAL CELLS
advertisement

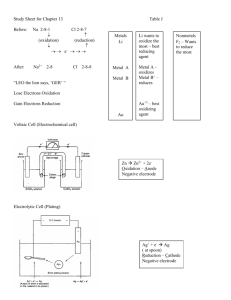
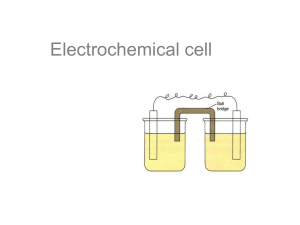
So far, Loss of electron(s) is oxidation while the gain of electron(s) is reduction. Reducing agent: a substance that loses or gives up electrons to another substance in a redox reaction. Oxidizing agent: a substance that gains or removes electrons from another substance in a redox reaction. Redox Table: Organizes metals in either increasing reactivity as an oxidizing agent or decreasing reactivity as a reducing agent. Pneumonics: LEO the lion says GER – LEO: loss of electrons is oxidation GER: gain of electrons is reduction “An Ox and Red Cat” – An Ox: Anode involves Oxidation Red Cat: REDuction involves CAThode The set up of an electrochemical cell is A B C – A: Anode B: Bridge C: Cathode ELECTROCHEMICAL CELLS (VOLTAIC) In cells of this type, spontaneous chemical change produces a flow of electricity. Oxidation occurs at the anode (attracts anion - ) Reduction occurs at the cathode (attracts the cation + ) Electron flow is always from the anode (-) to the cathode (+). An electric cell must have the following: 2 electrodes (solid conductor), an electrolyte (aqueous conductor) and a salt bridge (contains electrolyte as well). Electron flow can be measured in terms of voltage and current. Example: Cell Notation: Note: The substance with the lowest oxidation state is written closest to the electrode Standard Cell Potential (E) The E, the nought stands for standard conditions: 25C and 101.3 kPa, 1.0 mol/L Half-cell reduction potentials (voltages) can be determined by comparing them to the half potential for hydrogen half-cell. 2H+1 + 2eH2 E = 0.00V (arbitrary choice) A reaction is only spontaneous if the oxidizing agent is above the reducing agent in the Redox table. Using the electric potential values, if adding the two half-cells gives a positive value than the reaction is spontaneous as written. Consider the electrochemical cell example above: Example 2: Draw and label an electrochemical cell using Al/Al+3//Ni/Ni+2