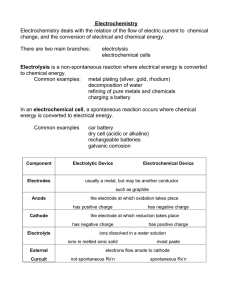
Electrolysis of Salt Water Lab
advertisement

Electrolysis of Salt Water Peter Lindner Ridgewood High School January 31, 2011 Today’s Setup - + V A My tests Voltage Current H2 Two dead 9Volts 7V 27mA OKAY Cell Phone Charger 5.1V 23mA OKAY Power Supply 34V 168mA GREAT Electrolyte Any substance containing free ions that make the substance electrically conductive Examples: • Sodium Chloride • Calcium Chloride • Citric Acid • Gatorade/Juices Questions • What are electrolytes? • Why is the electrolyte solution needed? • What is made at the Cathode? What is made at the Anode? • What are the benefits of this setup? When compared to the PEM electrolyzer? • What are the disadvantages? REACTIONS • Anode (positive): • Cathode (negative): OVERALL: 2H2O O2+4H++4e4H++4e-2H2 2H2O O2+2H2