CHM 2045L
CHM 2045L
Synthesis
Purpose: To synthesis an organic molecule commonly referred to as aspirin.
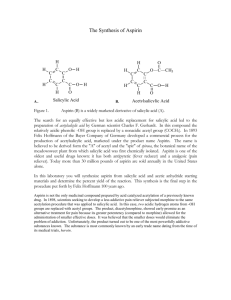
Background: One of the first drugs synthesized by chemist was aspirin. A scientist found that you could keep the pain relieving effect of salicylic acid but make it less damaging to the stomach by making chemically changing the acid to an ester. In this lab you will perform this simple, yet very important, reaction.
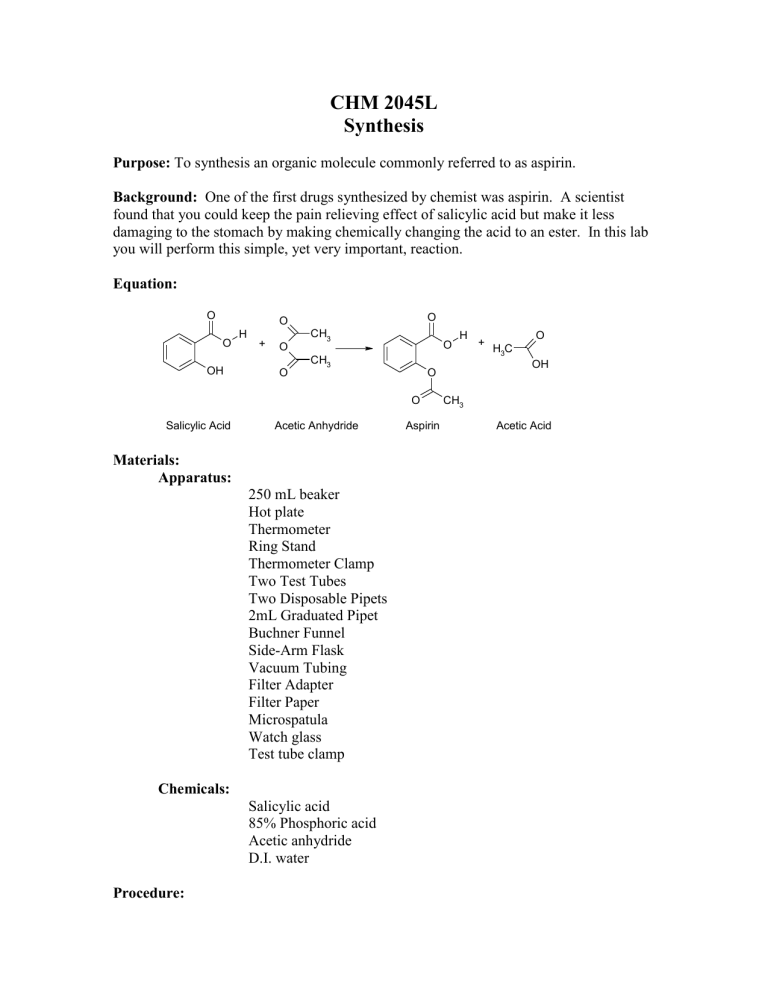
Equation:
O O
O
O
H
+
O
CH
3
O
H
+
H
3
C
O
CH
3
OH O O
OH
O CH
3
Salicylic Acid Acetic Anhydride Aspirin Acetic Acid
Materials:
Apparatus:
250 mL beaker
Hot plate
Thermometer
Ring Stand
Thermometer Clamp
Two Test Tubes
Two Disposable Pipets
2mL Graduated Pipet
Buchner Funnel
Side-Arm Flask
Vacuum Tubing
Filter Adapter
Filter Paper
Microspatula
Watch glass
Test tube clamp
Chemicals:
Salicylic acid
85% Phosphoric acid
Acetic anhydride
D.I. water
Procedure:
1.
Using a 250 mL beaker, set up a 95 o C water bath on a hot plate.
2.
Weigh out 1g of salicylic to the nearest 0.001g.
3.
Place the salicylic acid in a clean dry test tube.
4.
Add six drops of 85% phosphoric acid to the test tube.
5.
Add 1.8 mL acetic anhydride from a graduated pipet.
6.
Using a microspatula gently stir the contents in order to mix the reaction mixture
7.
Clamp the test to a ring stand and lower the reaction into the water bath
8.
The reaction mixture should completely dissolve.
9.
Heat the reaction mixture for 5 min., keep the temperature close to 95
10.
With a pipet slowly add 40 drops of water to the reaction mixture. o C.
11.
Remove the test tube from the water bath and allow it to cool to room temperature.
12.
As the reaction cool you should see the formation of a solid.
13.
If you do not see a solid form, ask your instructor to show you how to induce crystallization.
14.
Once the reaction mixture has reach room temperature, place it in a ice bath a cool it for 5 minutes.
15.
While the reaction mixture is cooling, also cool around 10 mL of D.I. water
16.
Record the weight of a piece of filter paper.
17.
Using a Buchner funnel set up a vacuum filtration system.
18.
Place the pre-weighed filter paper in the Buchner funnel, make sure to position the filter paper such that all the holes are covered
19.
Wet the filter with D.I. water
20.
Pour the reaction mixture onto the Buchner funnel and rinse it with 2mL portions of cool D.I. water
21.
After rinsing, allow the air to pull through the crystals for 3-5 minutes. You might need to break up the solid cake to allow airflow thru the solid.
22.
Carefully remove the filter paper with the crystals on it and place it on a watch glass.
23.
Place the product in your drawer to dry.
24.
Weigh out your product with the weigh paper.
25.
Subtract the weight of the weigh paper and record the weight of you Aspirin.
26.
Place solid aspirin in the waste aspirin container.
27.
Calculate the theoretical and percent yield of your product.
Pre-lab Questions:
1.
Calculate the molar mass of each of the following: a.
Salicyclic Acid ____________ b.
Acetic Anhydride ____________ c.
Aspirin ____________ d.
Acetic acid ____________
2.
Determine the theoretical yield of Aspirin based on 1.000g of Salicyclic Acid as the limiting reagent.
3.
When was Aspirin first synthesized? ______________
Source: ____________________________
4.
Why is the product rinsed with cold water (not room temperature water)?
Data Table:
Reactants:
Weight of Salicyclic Acid: ___________
Drops of 85% Phosphoric Acid: _________
Volume of Acetic Anhydride: __________
Theoretical yield based on Salicyclic acid as the limiting reagent:
(show your work)
Product:
Weight of Aspirin plus weigh paper: __________
Weight of weigh paper: __________
Weight of Aspirin:___________
Percent yield:___________
Post-lab Questions:
1.
What is the purpose of the 85% phosphoric acid?
2.
What would cause a percent yield to be over 100%?
3.
What is the purpose of the 40 drops of water added at step 10?
4.
Why is it important that the Aspirin be completely dry before obtaining a final weight?