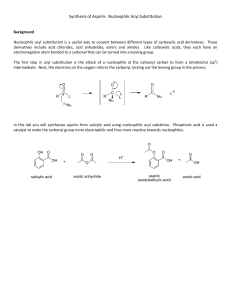
SCH 410: Techniques in Organic Chemistry LECTURE II, 2023 Separation of a mixture containing an acid, a base and a neutral compound in an organic solvent Extraction of the Basic Component • para-chloroaniline is basic 1, 4-dibromobenzene is neutral • Transfer the mixture to a separating funnel • Add dilute aqueous acid, HCl and shake, HCl converts the base to salt i.e. R- NH2 + HCl RNH+3Cl• Separate the aqueous layer containing the salt from the organic layer containing 1, 4dibromobenzene. • To the aqueous layer add conc. NaOH to convert RNH3Cl salt back to base i.e RNH+3Cl- + NaOH R- NH2 + H2O + NaCl • The base is then extracted from water using ether in a separating funnel. • Diethyl ether is then evaporated easily leaving the base behind, while NaCl remains in the aqueous layer. • The compounds are purified by crystallization and re- crystallization in organic solvent. Extraction of the Acidic Component from a mixture of a base, an acid and neutral substances • para-chloroaniline is basic 1, 4-dibromobenzene is neutral and benzoic acid is acidic. • Transfer the mixture to a separating funnel • A dilute inorganic base such as sodium hydroxide, sodium carbonate or sodium hydrogen carbonate is added to the mixture to converts organic acids to their sodium salts: RCOOH + NaOH → RCOO-Na+ + H2O OR RCOOH + NaHCO3 • → RCOO-Na+ +CO2 + H2O The acid is recovered by acidifying the aqueous layer with a strong acid. RCOO-Na+ + HCl → RCOOH + NaCl • The acid is recovered by extraction using diethyl ether, separating the two layers. • Diethyl ether is then evaporated easily leaving the acid behind, while NaCl remains in the aqueous layer. • The base can be extracted from the neutral compound as explained earlier. • If a mixture of phenol or naphthol and benzoic acid is to be separated, a strong base such as NaOH or Na2CO3 cannot be introduced into the mixture as both RCOOH and ROH are deprotonated. • A weak base, NaHCO3 is introduced first to selectively protonate RCOOH leaving behind the ROH in the ether layer. • A flow chart ca be used to outline the extraction of the various components from the mixture. However, the equations of the reactions cannot be displayed in the chart; therefore must be captured in the explanation of the chart. Extraction of caffeine from tea (Camellia sinensis) • Tea leaves are added to hot water and mixed thoroughly by stirring followed by filtration. The hot solution (filtrate) is allowed to cool • Caffeine is then extracted from the water using dichloromethane (methylene chloride), which is an organic solvent that is insoluble in water. Since caffeine is more soluble in dichloromethane (140 mg/ml) than it is in water (22 mg/ml), it readily dissolves in the dichloromethane (partitioning). • Tannins are slightly soluble in the dichloromethane but more soluble in water hence remain in water. • Sodium carbonate is then added to the mixture and the tannins and other phenolic compounds are converted to phenolic anions, which are not soluble in the dichloromethane but are soluble in highly polar water. Phenols are acidic and are converted to their salts (deprotonation of the -OH group) by reaction with sodium carbonate. • Caffeine is extracted into DCM by shaking the mixture in a separating funnel gently and not vigorously to prevent formation of emulsions by tannins. • The mixture is then allowed to settle before drawing the layer containing caffeine (organic layer). • Caffeine is obtained by evaporation of the DCM phase or crystallization. SYNTHESIS REACTIONS The following compounds are considered Aspirin Paracetamol Benzocaine Ibuprofen ➢ A synthesis reaction is a transformation of a substrate into desired product using appropriate reactants, reagents and/or catalysts under suitable conditions e.g. Temp, press, light, etc ➢ Single step synthesis: transformation of a substrate directly into the desired product without passing through intermediate steps. Example: Synthesis of aspirin (acetylsalicylic acid) To synthesize aspirin from salicylic acid, the ester functional group is created. Esters can be prepared by the reaction of a carboxylic acid with an alcohol (R-O-H). Preparation of aspirin by reacting salicylic acid with acetic acid Although the desired product is produced, this is an equilibrium reaction hence affecting the yield. The water produced reacts with aspirin to form salicylic acid and acetic acid. Preparation of aspirin by reacting salicylic acid with an excess of acetic anhydride. A small amount of a strong acid (phosphoric acid) is used as a catalyst which speeds up the reaction (esterification).Because no water is formed in the reaction the aspirin can’t hydrolyze (react with water) to reform salicylic acid. This reaction produces acetic acid as a second product instead of water. The excess acetic acid will be quenched with the addition of water. The aspirin product is not very soluble in water so the aspirin product will precipitate when water is added. Since acetic acid is very soluble in water, it is easily separated from the aspirin product. The aspirin isolated in this step is the “crude product”. A “purified product” can be obtained through recrystallization of the crude product in hot ethanol. The melting point range of pure aspirin is 138-140 0C and the melting point range of the salicylic acid starting material is 158-161 0C. (If impurities are present in the crude sample, the melting point range for the product will be lower than the range of pure aspirin). Single step Synthesis of Acetaminophen (paracetamol) OH OH O O O + + H3C NH2 O HO CH3 HN CH3 CH3 C O 4-aminophenol and acetic anhydride produces paracetamol (p-acetaminophen) and acetic acid. Preparation of acetaminophen involves treating an amine with an acid anhydride to form an amide. In this case, p-aminophenol, the amine, is treated with acetic anhydride to form acetaminophen (p-acetaminophen), the amide. Multistep synthesis: Syntheses reactions involving the conversion of reactants to products by following several steps. e.g. reactants are converted to intermediates which are then converted to products. Retrosynthesis Synthesis • Aspirin can be taken back to common chemical starting materials, ethanoic anhydride 5, phenol 8 and CO2 9. Propose a mechanism for the reaction between salicylic acid and the anhydride Retrosynthesis Synthesis Propose a mechanism for the reaction Retrosynthesis Synthesis Propose a mechanism for the reaction Retrosynthesis Synthesis Propose a mechanism for the reaction Synthesis Benzocaine is an anesthetic drug (numbing). It is used to reduce pain or discomfort caused by minor skin irritations, sore throat, sunburn, teething pain, vaginal or rectal irritation, hemorrhoids, or other sources of minor pain on body surface. CH3 CH3 + CH Cl AlCl3 3 benzene + HCl toluene COOH HNO3 Na2Cr2O7, H2O H2SO4 H2SO4/ Heat NO2 p-nitrobenzene Propose a mechanism for the reaction Sn/HCl/Heat NO2 O OH O O OH + H2SO4 95% ethanol NH2 p-nitrobenzoic acid p-aminobenzoic acid NH2 Benzocaine (ethyl aminobenzoate)