Synthesis of Aspirin
advertisement

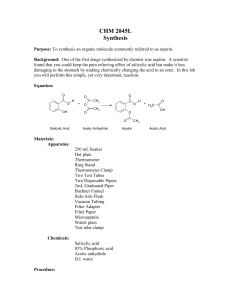
Name _________________________ Period _____ Date __________ SYNTHESIS OF ASPIRIN Purpose: The purpose of this experiment is to synthesize aspirin and to determine a percent yield. The purity of the aspirin will be found by determining the melting point of the compound and by the use of a salicylic acid indicator. Discussion: What is aspirin? Briefly describe the history of the development of aspirin. Write a general equation that exemplifies the reaction of salicylic acid with acetic anhydride. What is esterification? Write a mechanism for the synthesis of aspirin. Briefly describe the process of crystallization. What two purity tests will be used? Objectives: In this lab, you will… 1. synthesize aspirin through crystallization. 2. calculate the percent yield of aspirin. 3. assess the purity of the aspirin using melting point, a salicylic acid indicator, and thin-layer chromatography. Safety: Salicylic acid and aspirin may cause skin and eye irritation, but are basically non hazardous. Acetic anhydride and phosphoric acid can cause bad burns. Handle these chemicals with care, avoid breathing their vapors, and always wear safety goggles. Materials: Analytical Balance 10 mL graduated cylinder Hot plate 600 mL beaker Buchner funnel 1-hole rubber stopper Suction Erlenmeyer Rubber Hose Small Test Tube 250 mL Florence flask 50.0 mL graduated cylinder Thermometer Stirring rod 9.0 cm filter paper Aspirator Watch glass Test Tube Rack Melting Point Apparatus Chemicals: Salicylic acid Acetic anhydride Phosphoric acid Distilled water Ice Methanol 0.02 M FeCl3 Procedure: 1. Using the analytic balance, measure 3.00 g of salicylic acid and place it into a 250 mL Florence flask. 2. Measure 6.00 mL of acetic anhydride and add this to your flask. 3. Carefully add 5 to 10 drops of 85% phosphoric acid (the catalyst) to the flask and swirl gently for a few minutes to mix the components thoroughly. 4. Add ~200 mL of tap water to the 600 mL beaker. Place the reaction flask into the water, and heat it on a hot plate at 70˚C to 80˚C for 10 minutes. 5. After heating, remove the flask from the beaker and cautiously add 20 drops of distilled water. 6. Add 20.0 mL of distilled water and cool the reaction flask in an ice bath (600 mL beaker with ice water). If crystals do not appear, you can scratch the walls of the flask with a stirring rod to induce crystallization. 7. Assemble a vacuum filtration apparatus. 8. Mass a piece of 9.0 cm filter paper. Place the filter paper in the Buchner funnel and dampen it with distilled water. Turn on the water flow for the apparatus. 9. Vacuum filter the solid aspirin through the pre-massed filter paper. Wash the crystals with 2-3 mL of chilled water. Allow the air to be drawn through the solid and filter paper for 15 minutes. The liquid in the bottom of the flask is mostly water and can be washed down the sink. 10. Record the mass of a watch glass. Place the filter paper with the product on a watch glass. Place the watch glass on a piece of paper with you and your partner’s name on it. Place the watch glass in the drying oven. Your product will dry overnight. 11. After drying, mass the filter paper, aspirin and watch glass. 12. Check for impurities by dissolving a small sample of aspirin (~0.01g) in ~1 mL of methanol. Then drop by drop add 0.02 M FeCl3. The appearance of a purple hue indicates salicylic acid impurities in your aspirin. 13. Assess the purity of your aspirin product my measuring the melting point of the aspirin using the melting point determination procedure. Cleanup: Keep a small sample of your aspirin in a small labeled test tube for the next lab. Clean all glassware thoroughly. Put away any glassware you used. Analysis/Error: 1. Calculate the % yield of aspirin. 2. Calculate the % error using the melting points.