Aspirin Synthesis Lab Report: Chemistry Experiment
Synthesis of Aspirin Lab
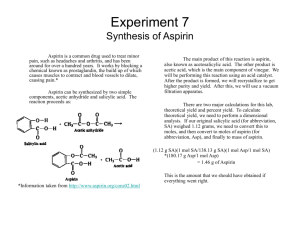
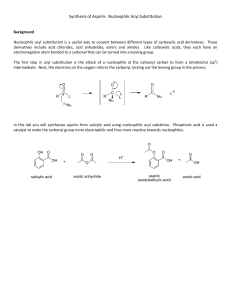
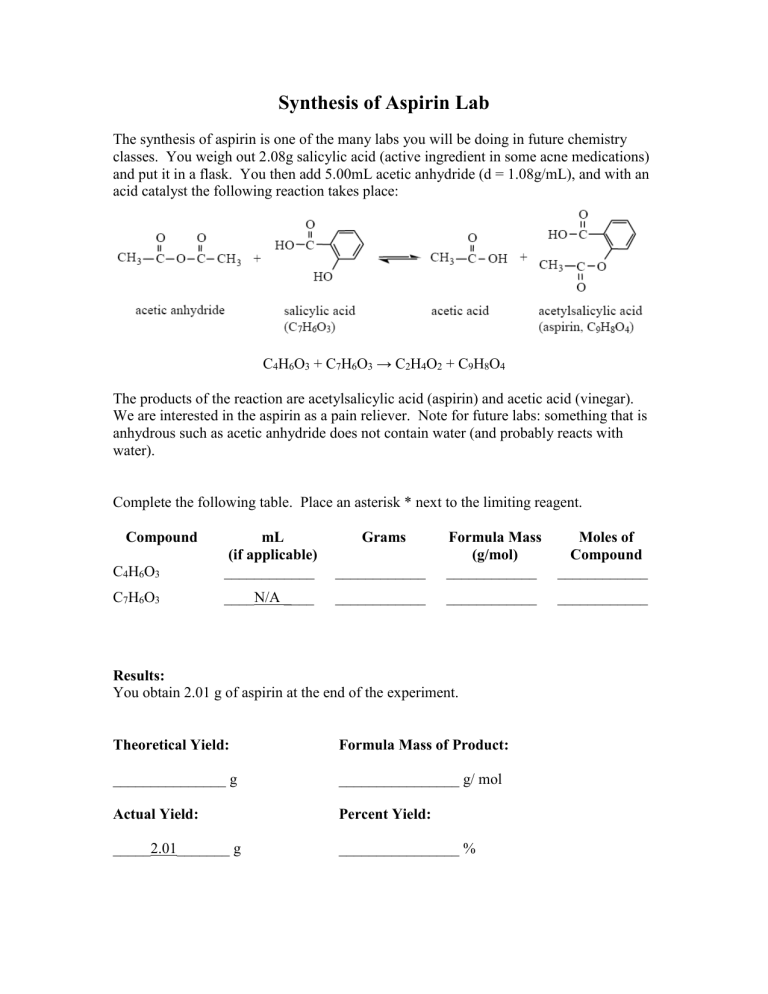
The synthesis of aspirin is one of the many labs you will be doing in future chemistry classes. You weigh out 2.08g salicylic acid (active ingredient in some acne medications) and put it in a flask. You then add 5.00mL acetic anhydride (d = 1.08g/mL), and with an acid catalyst the following reaction takes place:
C
4
H
6
O
3
+ C
7
H
6
O
3
→ C
2
H
4
O
2
+ C
9
H
8
O
4
The products of the reaction are acetylsalicylic acid (aspirin) and acetic acid (vinegar).
We are interested in the aspirin as a pain reliever. Note for future labs: something that is anhydrous such as acetic anhydride does not contain water (and probably reacts with water).
Complete the following table. Place an asterisk * next to the limiting reagent.
Compound
C
4
H
6
O
3 mL Grams Formula Mass Moles of
(if applicable) (g/mol) Compound
____________ ____________ ____________ ____________
C
7
H
6
O
3
____N/A ____ ____________ ____________ ____________
Results:
You obtain 2.01 g of aspirin at the end of the experiment.
Theoretical Yield:
_______________ g
Actual Yield:
Formula Mass of Product:
________________ g/ mol
Percent Yield:
_____2.01_______ g ________________ %