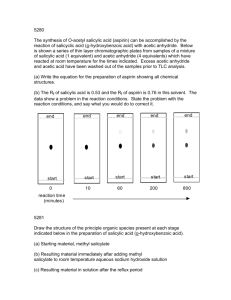
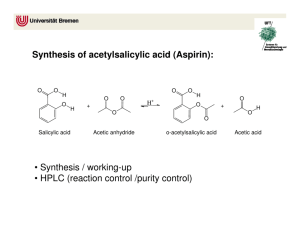
The Synthesis of Aspirin
advertisement
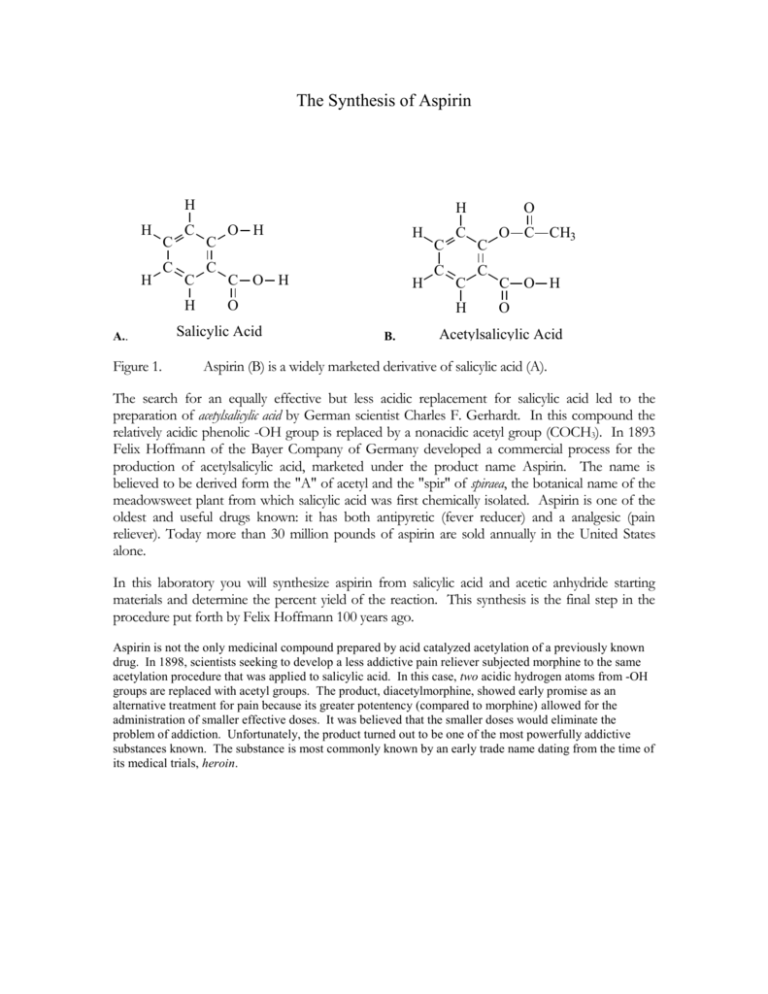
The Synthesis of Aspirin H H H C C C C H A.. Figure 1. H C C O H H C O H H O Salicylic Acid C C C C H B. O C C O C CH3 C O H O Acetylsalicylic Acid Aspirin (B) is a widely marketed derivative of salicylic acid (A). The search for an equally effective but less acidic replacement for salicylic acid led to the preparation of acetylsalicylic acid by German scientist Charles F. Gerhardt. In this compound the relatively acidic phenolic -OH group is replaced by a nonacidic acetyl group (COCH3). In 1893 Felix Hoffmann of the Bayer Company of Germany developed a commercial process for the production of acetylsalicylic acid, marketed under the product name Aspirin. The name is believed to be derived form the "A" of acetyl and the "spir" of spiraea, the botanical name of the meadowsweet plant from which salicylic acid was first chemically isolated. Aspirin is one of the oldest and useful drugs known: it has both antipyretic (fever reducer) and a analgesic (pain reliever). Today more than 30 million pounds of aspirin are sold annually in the United States alone. In this laboratory you will synthesize aspirin from salicylic acid and acetic anhydride starting materials and determine the percent yield of the reaction. This synthesis is the final step in the procedure put forth by Felix Hoffmann 100 years ago. Aspirin is not the only medicinal compound prepared by acid catalyzed acetylation of a previously known drug. In 1898, scientists seeking to develop a less addictive pain reliever subjected morphine to the same acetylation procedure that was applied to salicylic acid. In this case, two acidic hydrogen atoms from -OH groups are replaced with acetyl groups. The product, diacetylmorphine, showed early promise as an alternative treatment for pain because its greater potentency (compared to morphine) allowed for the administration of smaller effective doses. It was believed that the smaller doses would eliminate the problem of addiction. Unfortunately, the product turned out to be one of the most powerfully addictive substances known. The substance is most commonly known by an early trade name dating from the time of its medical trials, heroin. SAFETY NOTICE Safety Glasses must be worn at all times during the synthesis procedure. Phosphoric acid is toxic and corrosive. Causes severe irritation or burns. Avoid contact with skin or breathing of vapors. Extreme care should be taken when handling this material. Use only in a ventilation hood. Acetic anhydride is a corrosive and lachrymator. It is destructive to tissue of the mucous membranes and upper respiratory tract, eyes and skin. Use only in a ventilation hood. Salicylic acid causes irritation to the skin, eye, and respiratory tract. Avoid contact with skin or inhalation of dust. H H H C C C C H CH3 C C O H O C O + O C C O H CH3 O Salicylic Acid Acetic Anhydride H H H C C C C O C C H O C CH3 O + C O H H3C C OH O Acetylsalicylic Acid Acetic Acid Figure 2. Final step in the synthesis of Aspirin from salicylic acid and acetic anhydride. Materials: Salicylic Acid Acetic Anhydride Conc. Phosphoric Acid PSL – Extended Temp. Probe Beaker Filter Paper Hot Plate Ice Pipets Small Test Tube Procedure: 1. In a small test tube place 1.5 grams of salicylic acid. 2. Under the fume hood add 2 drops of phosphoric acid to the salicylic acid and 3 ml of acetic anhydride using the graduated pipet. Try to wash all other ingredients to the bottom of the tube. 3. Mix the reactants thoroughly and heat the mixture gently by placing it in a hot water bath. The temperature must be between 70 and 90 degrees. 4. When the reaction is complete (clear solution) remove the test tube. Cautiously add about .8 ml of distilled water to decompose any excess acetic anhydride. When you observe no more evidence of reaction add 1 more ml of water. Allow the test tube to slowly cool to room temperature. If crystallization does not occur spontaneously, add a seed crystal or scratch the inside of the tube with a stirring rod or gently tap the tube with your finger. 5. Cool the tube in a cup of ice water for several minutes until crystallization is complete. Do not let the tube spill over into the water. 6. Collect the crystals of acetylsalicylic acid by vacuum filtration with a Buchner funnel (see diagram below) and while filtering wash the product with tiny amounts of iced distilled water. 7. Obtain a piece of filter paper (label it as yours) and allow to completely air dry before recording its mass. Save your product in the assigned location for titration analysis, purity analysis and percent yield analysis. These will be undertaken as separate laboratories. Put the Buchner Funnel through the one-hole rubber stopper and then into the side-arm flask. Attach the rubber hose to the side-arm and also to the aspirator valve on the faucet.