DOC
advertisement
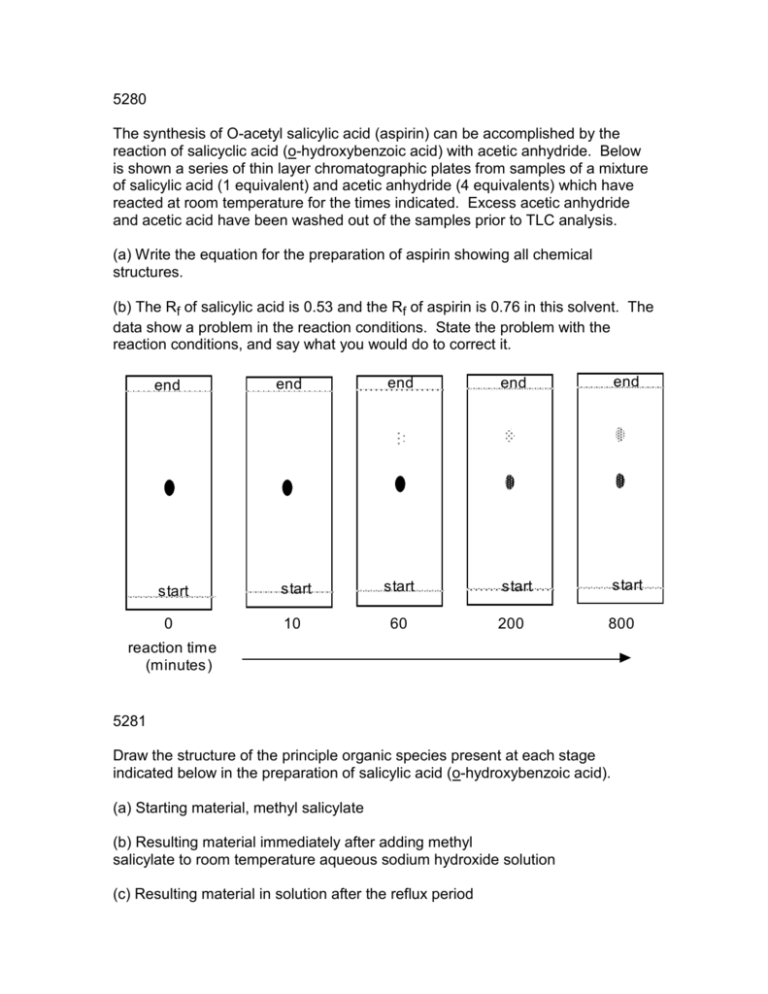
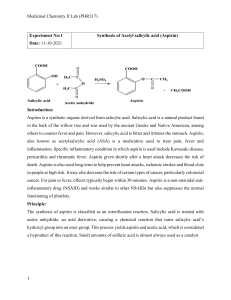
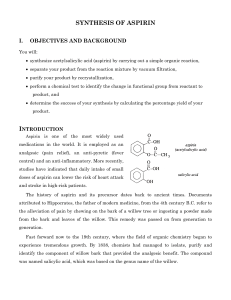
5280 The synthesis of O-acetyl salicylic acid (aspirin) can be accomplished by the reaction of salicyclic acid (o-hydroxybenzoic acid) with acetic anhydride. Below is shown a series of thin layer chromatographic plates from samples of a mixture of salicylic acid (1 equivalent) and acetic anhydride (4 equivalents) which have reacted at room temperature for the times indicated. Excess acetic anhydride and acetic acid have been washed out of the samples prior to TLC analysis. (a) Write the equation for the preparation of aspirin showing all chemical structures. (b) The Rf of salicylic acid is 0.53 and the Rf of aspirin is 0.76 in this solvent. The data show a problem in the reaction conditions. State the problem with the reaction conditions, and say what you would do to correct it. end start 0 end end end start start start start 10 60 200 800 end reaction time (minutes) 5281 Draw the structure of the principle organic species present at each stage indicated below in the preparation of salicylic acid (o-hydroxybenzoic acid). (a) Starting material, methyl salicylate (b) Resulting material immediately after adding methyl salicylate to room temperature aqueous sodium hydroxide solution (c) Resulting material in solution after the reflux period (d) Species which forms when the cooled reaction mixture is acidified with sulfuric acid