Grade 9 Academic Science – Chemistry
advertisement
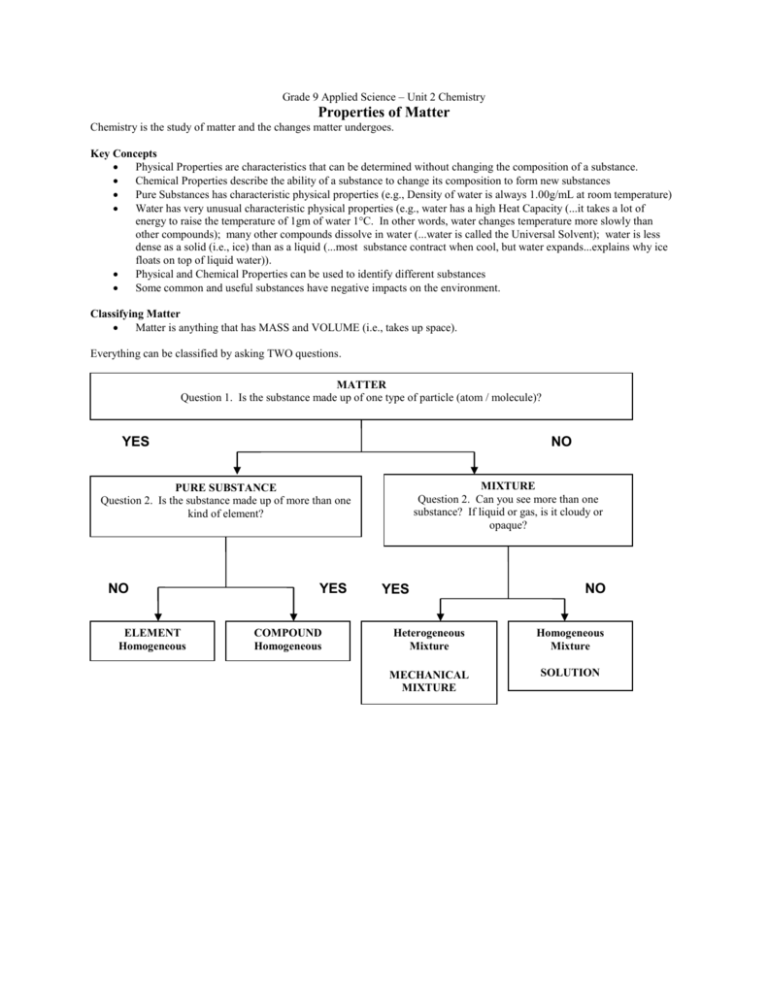
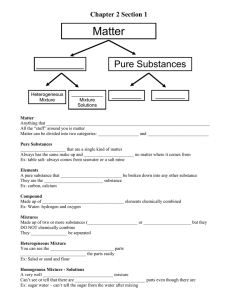
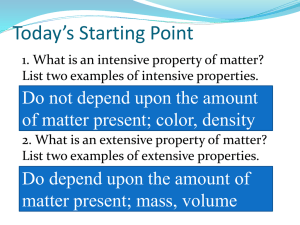
Grade 9 Applied Science – Unit 2 Chemistry Properties of Matter Chemistry is the study of matter and the changes matter undergoes. Key Concepts Physical Properties are characteristics that can be determined without changing the composition of a substance. Chemical Properties describe the ability of a substance to change its composition to form new substances Pure Substances has characteristic physical properties (e.g., Density of water is always 1.00g/mL at room temperature) Water has very unusual characteristic physical properties (e.g., water has a high Heat Capacity (...it takes a lot of energy to raise the temperature of 1gm of water 1°C. In other words, water changes temperature more slowly than other compounds); many other compounds dissolve in water (...water is called the Universal Solvent); water is less dense as a solid (i.e., ice) than as a liquid (...most substance contract when cool, but water expands...explains why ice floats on top of liquid water)). Physical and Chemical Properties can be used to identify different substances Some common and useful substances have negative impacts on the environment. Classifying Matter Matter is anything that has MASS and VOLUME (i.e., takes up space). Everything can be classified by asking TWO questions. MATTER Question 1. Is the substance made up of one type of particle (atom / molecule)? YES NO MIXTURE Question 2. Can you see more than one substance? If liquid or gas, is it cloudy or opaque? PURE SUBSTANCE Question 2. Is the substance made up of more than one kind of element? NO ELEMENT Homogeneous YES COMPOUND Homogeneous YES NO Heterogeneous Mixture Homogeneous Mixture MECHANICAL MIXTURE SOLUTION Use the information above, to write a definition in your own words. Table 1. Definitions of Matter Type of Matter Pure Substance Element Compound Mixture Mechanical Mixture Solution Definition