2013 Pure Substances and Mixtures
advertisement

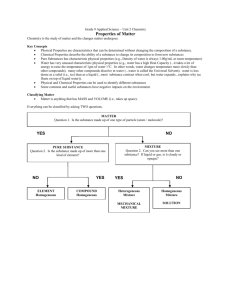
Today’s Starting Point 1. What is an intensive property of matter? List two examples of intensive properties. Do not depend upon the amount of matter present; color, density 2. What is an extensive property of matter? List two examples of extensive properties. Do depend upon the amount of matter present; mass, volume Today’s Starting Point 3. Compare these two elephants. What would be an intensive property of the elephants? What would be an extensive property of the elephants? By the end of the class period today I will be able to… Identify a piece of matter as an element, compound, homogeneous, or heterogeneous mixture based upon its properties Pure Substances Composition is the same throughout and does not vary from sample to sample. CANNOT be broken down by physical changes Can be an element or compound. Element Definition: substances in their simplest forms Cannot be broken down by a physical or chemical change Found on the periodic table Made up of one type of atom Examples of Elements: Hydrogen Carbon Lithium Gold What are two other examples of elements not listed above? What do all elements have in common? Compound Definition: substance formed by the chemical combination of two or more elements can be broken into simpler substances by a chemical change + = Compound Law of Definite Proportions A compound is always composed of the same elements in the same proportions. In other words, Carbon Dioxide (CO2) is always composed of 1 atom of C and 2 atoms of O. If there are different amounts of carbon or oxygen, it is no longer carbon dioxide. More Examples of Compounds Mixture Definition: two or more pure substances (elements or compounds) that are mixed together but NOT joined chemically NOT a pure substance Examples: The air we breath, gasoline for cars, the sidewalk on which we walk Homogeneous Mixtures Uniform in composition and appearance Same proportion of components throughout Consists of two or more substances in the same phase Also called solutions Heterogeneous Mixtures variable appearance and composition Raise ‘em Up! Look at the following example and with your partner determine if it is a heterogeneous mixture, homogeneous mixture, element, or compound Chicken Noodle Soup Pure Water Tap Water Pure Gold Coca-Cola Kool Aid has mass and takes up space Can be separated by allowing water to evaporate Same composition throughout