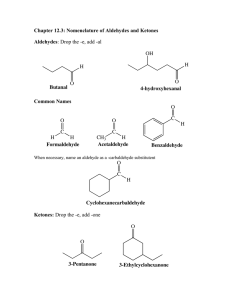
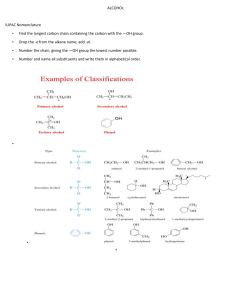
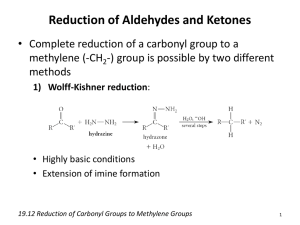
CHAPTER TWENTY-ONE: ALDEHYDES AND KETONES
advertisement

CHAPTER TWENTY-ONE: ALDEHYDES AND KETONES 1. Structure/ Properties o The carbon-oxygen bond o Acidity 2. Reactions at Carbonyl Carbon a. Nucleophilic Addition i. Oxygen Nucleophiles o Water to form hydrates a. Mechanism b. equilibrium o Alcohols to form Acetals or Hemiacetals a. Mechanism b. Equilibrium c. Dean-Stark Trap o Thioacetals as Protecting Groups ii. Nitrogen Nucleophiles o Ammonia, hydrazine, hydroxylamine and primary amines to form imines iii. Secondary amines to form enamines iv. Carbon Nucleophiles o Grigard reagents o Organolithium o Organocuprates and conjugate addition o Alkynyl anions o Cyanide anion to form cyanohydrin v. The Wittig reaction o Formation of Wittig reagent via SN2 between 1° alkyl halide and triphenylphosphine o Reaction of Wittig with ketone or aldehyde b. Reduction of aldehydes and ketones i. With Sodium borohydride in alcohol solvent ii. With Lithium aluminum hydride in dry solvent iii. Hydrogenation with hydrogen in Pd or Pt (alkenes are reduced under same conditions) o Selective reduction of alkene using Rh catalyst iv. Clemmenson reduction: reaction with Zn(Hg) in HCl to form alkane v. Wolff-Kishner reduction: reaction with NH2NH2 in KOH to form alkane including mechanism c. Oxidation of Aldehydes i. With Cr(VI) in aqueous soln o Chromic acid to carboxylic acid mechanism ii. With Ag(I): Tollens reagent (aldehydes only) iii. With PhCO3H: Baeyer-Villager oxidation to ester (not in Smith) LEARNING OUTCOMES: Predict the missing product and/or reactant in a relevant chemical reaction involving ketones and aldehydes. Use curved-arrow formalism to depict the known mechanism of an organic reaction. Devise multi-step syntheses of molecular targets using known reactions. Understand the structural features that lead to product selectivity in selective organic reactions. SAMPLE EXAM PROBLEMS: 1. Circle the symbol that best depicts the position of equilibrium for the following reversible reaction? O OH H2O + OH K > < - 1 2. Propose a sequence of reactions to synthesize the following target from benzene: O