Reduction of Aldehydes and Ketones
advertisement

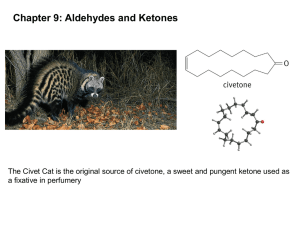
Reduction of Aldehydes and Ketones • Complete reduction of a carbonyl group to a methylene (-CH2-) group is possible by two different methods 1) Wolff-Kishner reduction: • Highly basic conditions • Extension of imine formation 19.12 Reduction of Carbonyl Groups to Methylene Groups 1 Reduction of Aldehydes and Ketones 19.12 Reduction of Carbonyl Groups to Methylene Groups 2 3 4 Reduction of Aldehydes and Ketones 2) Clemmensen reduction: • Acidic conditions • Zinc amalgam + HCl • The mechanism is uncertain 19.12 Reduction of Carbonyl Groups to Methylene Groups 5 6 Problems 1) Draw the products for the following reactions: 7 2) Draw the complete mechanism for the following reaction: 8 The Wittig Alkene Synthesis • Method of preparing alkenes from aldehydes and ketones 9 • Regioselective rxn, assuring the location of alkene 10 The Wittig Alkene Synthesis • Preparation of phosphorous ylide • Ylid (or ylide): compound with opposite charges on adjacent, covalently bound atoms 19.13 The Wittig Alkene Synthesis 11 12 The Wittig Alkene Synthesis • Stereochemistry – Both E and Z products • Retrosynthetically 13 14 Problems 1) Draw the product(s) for the following reaction 2) What carbonyl compound and phosphorus ylide might you use to prepare the following compound? 15