Chapter 12.3: Nomenclature of Aldehydes and Ketones Aldehydes Butanal 4-hydroxyhexanal
advertisement

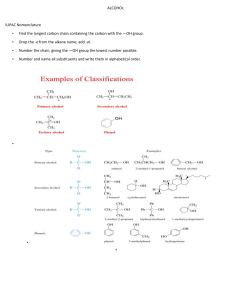
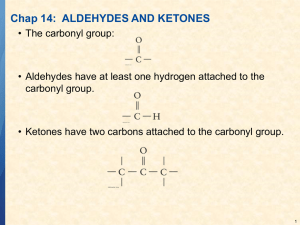
Chapter 12.3: Nomenclature of Aldehydes and Ketones Aldehydes: Drop the -e, add -al OH H H Butanal O 4-hydroxyhexanal O Common Names O C O O C H H Formaldehyde C CH3 H Acetaldehyde H Benzaldehyde When necessary, name an aldehyde as a -carbaldehyde substitutent O C H Cyclohexanecarbaldehyde Ketones: Drop the -e, add -one O O 3-Pentanone 3-Ethylcyclohexanone Common Names: O O O O Acetone Methyl ethyl ketone (MEK) Benzophenone Acetophenone If aldehydes or ketones need to be named as substituents, use the term "oxo": O H O 4-oxopentanal When referring to the location of substituents relative to a carbonyl group, organic chemists sometimes use a Greek letter (α, β, γ, etc.). This is not normally used in formal naming, but is often used when talking about classes of molecules. Cl O O C # C " C ! O H R C R OH A "-ketoacid O An !-chloroaldehyde Multiple functional group priority When naming polyfunctional compounds, the functional group with the highest priority goes into the root name, the other groups are named as substituents. The order of priority generally follows oxidation level, more highly oxidized groups have higher priority: acid > acid derivatives > aldehydes > ketones > alcohol > amine > ether Double and triple bonds are included in the root name. O O OH 4-Hydroxy-2-pentanone NOT 4-oxo-2-pentanol CH3 C C C CH3 3-Pentyn-2-one