Chem 242 Aldol Condensation
advertisement

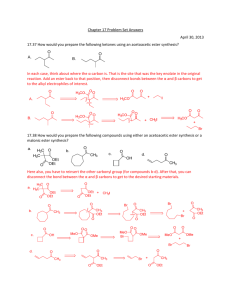
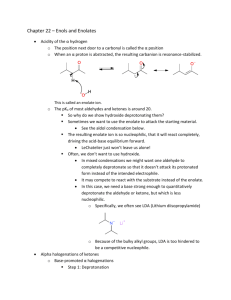
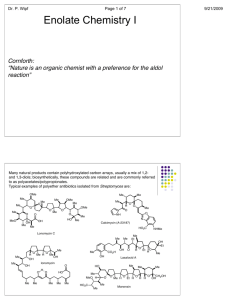
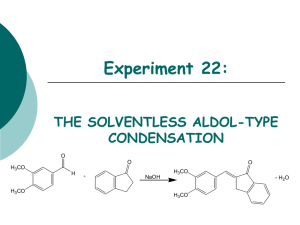
Chemistry 242 Exp. 4 Solventless Aldol Condensation We talked some about “green chemistry” last term. This lab is another “green” procedure in that it uses no reaction solvent even though the reactants are all solids. This saves expenses in terms of both the initial purchase of the solvents and their disposal. In addition, there is no need to separate the product from the reaction solvent, because there isn’t any. We still use solvent for recrystallization, though. The placement of this lab presents a bit of a quandary. It could come towards the beginning of the organic chemistry lab (start of 241) because it illustrates the depression of mixture melting points: mixing two solids together and having them become a liquid solution (because their mixture melting point was depressed to below room temperature). Or, we could wait until the third term when we study carbonyl compounds. Alternatively, we could take a middle route, covering this lab after not only melting points, but after mechanisms, too, so we can understand what is happening in the reaction. I chose this third route, because it is kind of short and may allow some catch up time. This will serve as a good reminder of mixture melting point behavior and we have recently studied enol mechanisms, so this is a good time to have this lab. I don’t expect you to be able to reproduce this mechanism yet (not until chemistry 243), but I do expect you to follow along with it. It has a couple of concepts that are relative to a lot of organic chemistry reactions we have studied so far: nucleophile/electrophile attractions and acid-base chemistry. Alpha Hydrogens to a carbonyl are acidic because the product conjugate base is resonance stabilized. “Alpha” is just a way of counting from a functional group (or other designated point in the molecule). Alpha is one carbon away, beta is two, gamma is three, and so forth. The conjugate base product of an acid/base reaction with these acidic hydrogens is called an enolate ion. The “en” from alkene and the “ol” from alcohol. This part is similar to the enols we have been studying, but with a negative charge on a carbon adjacent to the carbonyl, hence the “ate ion” on the end of the term. In the first step of this reaction, the initial reaction with base removes a proton from the alpha carbon creating a deprotonated enol, hence the name enolate ion. Step 1. Formation of the enolate ion OH O H C H2 O O CH2 CH2 resonance for enolate ion In the next step, the enolate acts as a nucleophile, adding to the carbonyl carbon of another molecule. If the carbonyl the enolate adds to is a different molecule, it is 1 called a “crossed aldol” reaction. If the enolate adds to a molecule of itself that has not been deprotonated yet, it is called a “self aldol” or “homo aldol” reaction. A deprotonated enolate ion will not add to another enolate ion. The negative charges repulse each other too much. Step 2. Addition of the enolate to a carbonyl carbon. In this example, if both R’s = -CH3, then this is a self aldol. If either or both R groups are anything other than methyl groups, this is a crossed aldol. O R O O O CH2 R R R This addition product is treated with acid to give a β-hydroxyketone (hydroxy is the substituent name for an alcohol – see Bruice, p. 791). Step 3. O H O OH2 O OH R R R R The β-hydroxyketone can then undergo a condensation reaction, eliminating water. This condensation reaction is not reversible and is driven by the conjugation of the ketone and alkene pi bonds. It goes even more readily if the R groups are also conjugated with the emerging alkene, such as benzene rings would be. Step 4. O H OH R OH2 O R R R H HOH 2 To avoid all the different possible products, usually only one of the two carbonyl compounds has alpha hydrogens. Otherwise, the number of different crossed aldols and self aldols makes isolation of the desired product difficult and also lowers the yield of the desired product. See page 874 in Bruice for more on aldol reactions. The reactants in this experiment will be 3,4 dimethoxybenzaldehyde and 1indanone. O O O + NaOH OMe H OMe OMe OMe 3,4-dimethoxybenzaldehyde product Me = methyl mp = 180 C Safety precautions: Although green chemistry reduces safety precautions, it is difficult to eliminate them all together. Solid sodium hydroxide is a very strong base. Skin contact should be avoided. NaOH is also very hygroscopic (readily picks up moisture from the air. Close the NaOH container lid IMMEDIATELY after removing your solid product and BEFORE doing anything else. This is VERY IMPORTANT. This is a synthesis, and the procedure for synthesis prelabs will be followed again. You need a balanced reaction showing stereochemistry and a table of reactants, like the one below: reagent mw (g/mole) 166 mL used - density 132 - - NaOH 40.0 - - product 280.0 - - 3,4 dimethoxybenzaldehyde 1-indanone Limiting reagent g used moles used - - mole ratio theor actual - ________________________________________________ Topics to review: aldol reactions (preview, actually), recrystallization, melting pts 3 Reaction Instructions: 1. Place 0.25 g of 3,4-dimethoxybenzaldehyde and 0.20 g of 1-indanone in a test tube. Use a stirring rod or metal spatula to scrape and crush the two solids together until they become a brown oily mixture. Remember that your strength and enthusiasm may exceed the beaker’s limits: don’t push out the bottom of the beaker. Do not continue until step two until all the solid is gone. This may take as long as 20 minutes, but if not done, you may have to start all over again. 2. Add 0.05 g of finely ground (using mortar and pestle) solid NaOH to the reaction mixture and continue scraping until the mixture becomes solid. This much NaOH is less than one pellet; you can easily share with fellow students to avoid waste. Isolation: 3. Allow the mixture to stand for 15 minutes, then add about 2 mL of 10% HCl solution in water. Scrape your product off the walls of the beaker to expose it to the HCl. Check the solution pH with 1 cm or smaller pieces of paper to be sure that it is acidic (less than 7). Remember to record exactly how much HCl is used as you would with anything that you add to your reaction. The whole point of science is to reproduce your results. If you do not know what and how much you added of each ingredient, you cannot possibly expect to reproduce your results with accuracy. 4. Isolate the solid with vacuum filtration. Let the solid stand in the funnel with the water drying to pull air through in order to dry the product a little. 5. Recrystallize the product with a 90% ethanol/10% water mixture, first using some hot solvent to rinse any remaining product out of the beaker. Remember that recrystallizations use already hot solvent. Add only barely enough hot solvent to get your product dissolved or your yield will be greatly reduced. You should not need more than 20 mL of solvent. After dissolving, let cool to room temperature, then place in an ice bath. 6. Filter product crystals and spread them out on your watch glass with a scrap of paper (weighing paper does nicely for this) so that the crystals can dry for a week before doing step 7. Characterization. 7. Determine the mass of your product and its melting point Post lab analysis: Analyze purity and yield of your product. In other words, what was your percent yield? Do you think that is good or bad? If your percent yield is poor, what do you think happened that reduced your yield? What do you think you could do next time to improve your yield? Did the color and mp of your product match the literature mp and color? Was it even close? Post Lab Questions: 1. In this reaction, there is only one carbon with acidic alpha Hydrogens. Draw the reactant with it and circle the carbon with the acidic Hydrogens. 4 2. What are the products of these crossed aldol condensation reactions (only one possible answer)? O O + (H3C)3C C(CH3)3 O O + O O + 3. In other reactions, both reactants may have acidic Hydrogens, leading to two different products. What are the two products of these crossed aldol condensation reactions? O O + O O + O O + 5