Chapter 22 - People.vcu.edu
advertisement

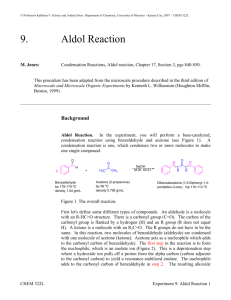
Chapter 22 – Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonance-stabilized. - This is called an enolate ion. o The pKa of most aldehydes and ketones is around 20. So why do we show hydroxide deprotonating them? Sometimes we want to use the enolate to attack the starting material. See the aldol condensation below. The resulting enolate ion is so nucleophilic, that it will react completely, driving the acid-base equilibrium forward. LeChatelier just won’t leave us alone! Often, we don’t want to use hydroxide. In mixed condensations we might want one aldehyde to completely deprotonate so that it doesn’t attack its protonated form instead of the intended electrophile. It may compete to react with the substrate instead of the enolate. In this case, we need a base strong enough to quantitatively deprotonate the aldehyde or ketone, but which is less nucleophilic. o Specifically, we often see LDA (Lithium diisopropylamide) o Because of the bulky alkyl groups, LDA is too hindered to be a competitive nucleophile. Alpha halogenations of ketones o Base-promoted α halogenations Step 1: Deprotonation LDA - Step 2: Enolate attacks the halogen LDA - This reaction keeps going, because any additional α protons are even more acidic than they were to begin with. o Haloform reaction A test for methyl ketones When a ketone has a methyl group, the resulting –CX3 is a good enough leaving group to be replaced by the hydroxide. Overall, a methyl ketone reacts with X2 and hydroxide to give a carboxylate and a haloform (CHX3) o Acid-catalyzed α halogenations Easier to add just one halogen Alkylation of enolate ions o SN2 is back again! NaOH - - Aldol condensation o Enolate ion attacks a carbonyl H+ - o If you heat the product up, it loses water to form a new carbon-carbon double bond. A common question in this material is when you’ll lose water and when you won’t. First, calm down. This material is on the take-home quiz and a few questions on the ACS, so it’s less tested than the rest of the semesters. Next, Mr. Baker’s take-home will make it clear when to do this. o Either heat will be indicated with the arrow (as below). o Or the resulting product will be highly conjugated, driving the reaction forward. The ACS is multiple choice! o Most likely, both products will not be shown. o If both products are shown, I personally would choose the dehydrated product, but as I don’t look at this test I can’t tell you what to do here. heat o Crossed or mixed aldol condensations This just means that you started with two different ketones or aldehydes. Claisen Condensations o Similar to aldol, but with esters you get nucleophilic substitution at the carbonyl instead of nucleophlic addition at the carbonyl. o The drawing below shows the overall reaction, but remember that with nucleophilic substitution at the carbonyl there is always a tetrahedral intermediate. NaOEt EtOH o Why does this become favorable? - The product deprotonates because the hydrogens which are α to both carbonyls are even more acidic (pKa about 12) Malonic ester synthesis o Malonic ester A hydrogen α to two carbonyls will be even more acidic than a regular α hydrogen. In this case, the pKa is 13 o First, the malonic ester is deprotonated. NaOEt - o Then, the enolate ion attacks an alkyl halide in an SN2 reaction. - o The esters undergo hydrolysis. H3O+ o When heated, any carboxyl group that is β to a carbonyl will decarboxylate. heat 2 1 This is called decarboxylation of β-keto acids Overall, the COOH turns into a H. o Again, be flexible in your thinking. This is just one example of a reaction, but the individual steps can all happen with different chemicals. Michael Addition o When a pi bond is conjugated with a carbonyl, there are two electrophilic sites. - - o Because of this, nucleophiles can add to either partially positive site. o When a nucleophile adds to the carbon-carbon double bond, this is called Michael Addition. o How do you know whether a nucleophile will add to the alkene or to the carbonyl? The really strong nucleophiles add to the carbonyl. Grignards and alkyl lithiums The other nucleophiles add to the carbon-carbon double bond. Enolate ions Dialkyl cuprates (R2CuLi) (CH3)2CuLi Robinson annulations o First, you do a Michael addition using an enolate ion as your nucleophile. - o If a cyclic aldol reaction would yield a six-membered ring, then do it! OH Base 1 2 2 1 1 3 3 3 4 4 - 6 6 7 7 o Finish it off with a dehydration. OH 1 6 2 3 5 4 7 5 4 5 5 6 2 1 6 2 3 5 4 7 7