Enolate Chemistry I
advertisement

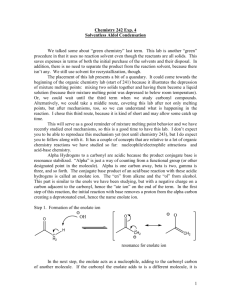
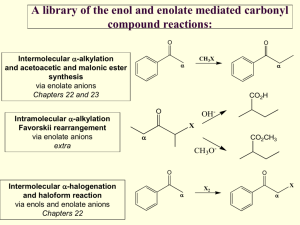
Dr. P. Wipf Page 1 of 7 9/21/2009 Enolate Chemistry I Cornforth: “Nature is an organic chemist with a preference for the aldol reaction” Many natural products contain polyhydroxylated carbon arrays, usually a mix of 1,2and 1,3-diols; biosynthetically, these compounds are related and are commonly referred to as polyacetates/polypropionates. Typical examples of polyether antibiotics isolated from Streptomyces are: OMe Me Me O Me Me MeO Me Me O O O O OMe Me Me O HO OH OH Me Me Me O H OMe O Me O N Me Calcimycin (A-23187) HO2C Me Me Me H Me H O OH Me O Me H Me Me OH O O HO Me CO2H OH Ionomycin OH Me Me NH Me Lonomycin C O O O Me Me HO2C H Me O Me Me O O OH Et Et H O Me Lasalocid A HO Me MeO H Me Et Me OH NHMe O Me H O Et H Monensin Me O H H Me O CH2OH OH Dr. P. Wipf Page 2 of 7 9/21/2009 Most of these compounds are metal chelators (ionophores). Monensin as a food additive kills bacteria in poultry by the transporting Na+ outside the cell, thus increasing the intracellular osmotic pressure and causing the coccidia to explode. Macrolide antibiotics are characterized by the macrocyclic lactone moiety and can be grouped into: polyoxo, polyene, ionophore, and ansamycin macrolides. O O HO Me O Me HO Me Me O O Me Me Me Me O OR Me Me Me HO Me OR O Me O O Me Methymycin R = desoaminyl OR OR' O Me Pikromycin R = desoaminyl Erythromycin A R = desoaminyl R' = cladinosyl O O Me Me Me CHO MeO O Me OH Me Me OH Me O CHO R'O OR O O OH Me OR OH Me Tylosin R = mycarosyl-mycaminosyl R' = mycinosyl Leucomycin A1 R = mycarosyl-mycaminosyl OH OH OH O HO O OH OH OH OH O OH O OH HO O OH OH OH OH O CO2H OH O NH2 OH CO2H O O Nystatin A1 OH Amphotericin B OH O NH2 OH Dr. P. Wipf Page 3 of 7 9/21/2009 O O H O O H O H O H H O H O H O O H O O Nonactin Ac OH Me OH H N Me Cl Me O O O MeO OCH2CO2H O HO Me Me Me O O O N Me Me Me Me O N OMe OAc OH O OH OMe H Me Maytansine Rifamycin S O O Bistramide C N Me O O Me H N O HO HN O O O H H 13 5 9 O MeO O H H O O 1' O 1 5' O 17 7' O 9' HN O N OMe Leucascandrolide A Dr. P. Wipf Page 4 of 7 9/21/2009 1. Introduction O O R2C C R2C R' C H R' H α proton is relatively acidic (pKa≈20); it can be removed by bases carbonyl group is electrophilic; nucleophilic reagents add to carbonyl group α carbon atom of enol is nucleophilic; it attacks electrophilic reagents Carbonyl compounds exist in equilibrium with their tautomers, which are enols. They can express a variety of different kinds of chemical reactivity. The enol tautomer is quite reactive toward electrophiles, and the above equilibrium rapidly regenerates new enols in the presence of acid or base catalysts. However, most reactions of electrophiles with carbonyl compounds take advantage of the higher nucleophilicity of the deprotonated enol, the enolate anion. When unsymmetrical carbonyl compounds are converted to their enolates under conditions that allow equilibrium to be established (high temperatures and weak bases) the enolate anion with the more highly substituted double bond is formed. This ion is the more stable of the two possible enolates and is called the thermodynamic enolate. If the carbonyl compound is added to an excess of a strong base (usually LDA) at low temperatures, the least hindered hydrogen atom is removed. The ion with the less substituted double bond forms; it is the less stable of the two possible enolates but is the one that forms faster. For this reason, it is called the kinetic enolate. Dr. P. Wipf Page 5 of 7 9/21/2009 Reactions of aldehydes and ketones that involve enol or enolate ion intermediates include: Enolization. Aldehydes and ketones exist in K equilibrium with their enol forms. The rate at OH O which equilibrium is achieved is increased by K = 1x10-8 acidic or basic catalysts. The enol content of simple aldehydes and ketones is quite small; β-diketones, however, are extensively enolized. α-Halogenation. Halogens react with aldehydes and ketones by substitution; An acid catalyst (or base) increases the rate of enolization (enolate formation), which is the rate-determining step. The reaction of a methyl ketone with a halogen in base is known as the haloform reaction. Once one of the α-H's is replaced by a halogen atom, the remaining H's are more acidic and are more easily substituted. C,C-bond cleavage is facilitated by the e-withdrawing effect of the halogens. Aldol condensation. A reaction of great synthetic value for C,C-bond formation. Nucleophilic addition of an enolate ion (or an enol in the acid-catalyzed version of this process) to a carbonyl group leads to a β-hydroxy aldehyde or ketone (reversible!). Subsequent dehydration (acid- or base-catalyzed) yields an α,β-unsaturated carbonyl compound. O RCH2 C HO NaOCH2CH3 R' HOCH2CH3 RCH2 (2 equiv) O O R' R' R LDA O R' NaOCH2CH3 HOCH2CH3 -H2O RCH2 O OLi Li CH3CH2CHO O R' R THF, -78° C CH2CH3 H2O O OH 85% (workup) CH2CH3 The irreversible, rapid enolization of carbonyl compounds with very strong base (LDA), followed by addition of aldehydes or ketones, allows the direct formation of a single aldol product in crossed aldol condensations. Dr. P. Wipf Page 6 of 7 9/21/2009 Claisen-Schmidt reaction. A crossed aldol condensation in which a non-enolizable aldehyde reacts with an enolizable aldehyde or ketone. O Ph C O H + (CH3)3C C HO NaOH CH3 O Ph HOCH2CH3 H2O C(CH3)3 H H NaOH O 90% HOCH2CH3 -H2O C(CH3)3 Ph Robinson annulation. A combination of conjugate addition of an enolate anion to an α,βunsaturated ketone (Michael addition) with subsequent intramolecular aldol condensation. Michael additions are typical for soft nucleophiles, including enols, cyanides, cuprates and thiol(ate)s. In contrast, hard nucleophiles (hydrides, Grignard and lithium reagents) prefer 1,2-addition. Alkylation reactions. Treatment of enolates with halides provides a means for carbon chain extensions at the α-position. However, polyalkylation can be a problem, and the use of a strong, nucleophilic base or the enamine is recommended. O O 1. pyrrolidine, benzene 2. butyl iodide 3. 10% H2SO4 O H pyrrolidine, benzene H N + TsOH 85 : 15 N Dr. P. Wipf Page 7 of 7 9/21/2009 Aldol Methodology Synthetic problem: control of aldol regio- and stereoselectivity. O OH O OH + Ph O Ph OH O Ph O + O + OH Ph base + enantiomers O Ph O OH O Ph + Ph + OH Ph OH O OH Ph Woodwardʼs synthesis of cortisone (Woodward, R. B. et al. J. Am. Chem. Soc. 1952, 74, 4223): O O OH OH H H H O cortisone Target molecule: 6 chiral centers, 64 possible stereoisomers. This synthesis illustrates, rather ingeniously, the selective manipulation of functionalities on six-membered rings, and a vastly increased synthetic arsenal.