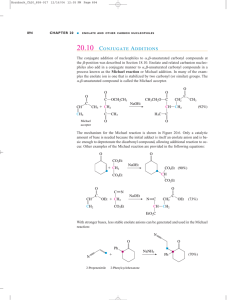
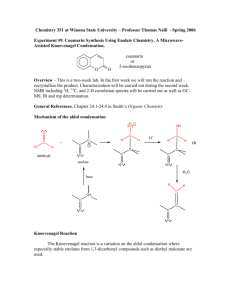
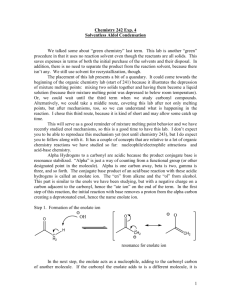
Prelab for “Synthesis of 2-Methyl-2
advertisement

Prelab for “Synthesis of 2-Methyl-2-pentenal: An Aldol Condensation” Name: Section: 1. Propane has pKa1 near 50, as compared to pKa of 20 for propanal (the protons on the CH2). Which compound is more acidic and by how much (the ratio of Ka2/Ka1 NOT pKa2/pKa1)? 2. Draw an equation showing the loss of a proton from CH2 carbon in propanal. Examine the anion formed and draw the other important resonance structure. 3. Draw the structure of 3-hydroxy-2-methylpentanal. When this compound dehydrates upon heating, it has the choices of eliminating a molecule of water to form either 2-methyl-2pentenal or 2-methyl-3-pentenal. Explain why the former is favored (hint: consider the position of the new C=C and stability of each product). 4. Draw a balanced equation describing the proton removal by NaOH from the CH2 proton in CH3CH2CH=O to form its enolate (consult the first part of Chapter 22 of Wade). What would the resulting enolate anion react as, a nucleophile or an electrophile? If the enolate is allowed to react with CH3CH2CH=O, which carbon in the propanal molecule will the enolate anion attack?