Expt 9. Knoevenagel Condensation. Synthesis of Coumarin
advertisement

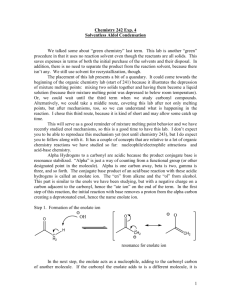
Chemistry 351 at Winona State University – Professor Thomas Nalli - Spring 2006 Experiment #9. Coumarin Synthesis Using Enolate Chemistry. A MicrowaveAssisted Knoevenagel Condensation. O O coumarin or 2-oxobenzopyran Overview – This is a two-week lab. In the first week we will run the reaction and recrystallize the product. Characterization will be carried out during the second week. NMR including 1H, 13C, and 2-D correlation spectra will be carried out as well as GCMS, IR and mp determination. General References. Chapter 24.1-24.4 in Smith’s Organic Chemistry Mechanism of the aldol condensation O O C + C R OH H aldehyde C R H H+ C R H C C O enolate C C C O O -H2O base R H C CH C C O C O Knoevenagel Reaction The Knoevenagel reaction is a variation on the aldol condensation where especially stable enolates from 1,3-dicarbonyl compounds such as diethyl malonate are used. (2) O C C O O C C C O O C C O C H H stable enolate from a 1,3-dicarbonyl compound C H These stable enolates are cleanly and catalytically formed by reaction of the dicarbonyl compound with a weak base such as piperidine. piperidine, a secondary amine N H In the synthesis of a coumarin compound using the Knoevenagel reaction, an ohydroxybenzaldehyde is reacted with a stable enolate. The Knoevenagel (or aldol) condensation initially forms a diester, which then undergoes transesterification with the ortho hydroxy group to form the coumarin ring plus ethanol. O N O H R1 3 + OH EtO R 3 R H R1 R2 R2 O OH OEt + H 2O R3 + EtOH 1 R O O 2 R Reaction Procedures Use 0.010 mol of the hydroxyaldehyde (salicylaldehyde, o-vanillin or 4diethylaminosalicylaldehyde). Calculate the amount of dicarbonyl compound (diethyl malonate or ethyl acetoacetate) to use based on a 1:1 stoichiometry figuring in a 10% excess of the ester. Mix the two reactants in a small vial and add 0.04 g piperidine. (Piperidine functions as the base in the reaction – it reacts with diethyl malonate to form the enolate ion.) Place the vial, loosely covered in the microwave oven and irradiate for 2-3 min at 30% power. Remove and immediately check the temperature of the solution using an IR thermometer. Repeat the microwave irradiation if necessary until the reaction mixture’s temperature reaches that specified in the literature reference. After cooling to room temperature the crude solid is recrystallized from ethanol/water to afford a pure final product. This is a mixed solvent recrystallization. Dissolve the solid in the minimum amount of hot ethanol then add water slowly until the solution remains slightly cloudy even at the boiling point. Now add enough ethanol to make it clear up again then cool slowly to allow crystals to grow. Collect the crystals by vacuum filtration and wash with a small amount of cold 1:1 ethanol:water. Reference http://www.ch.ic.ac.uk/ectoc/echet98/pub/o87/index.htm