spWS8
advertisement

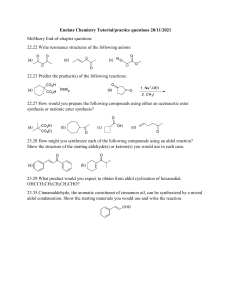
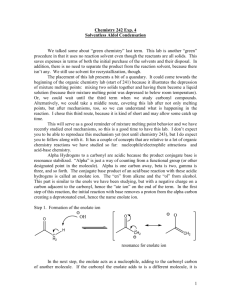
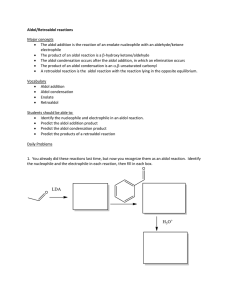
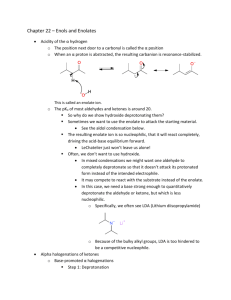
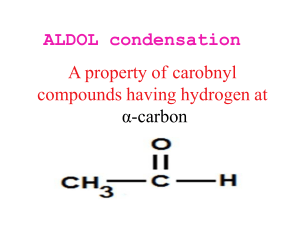
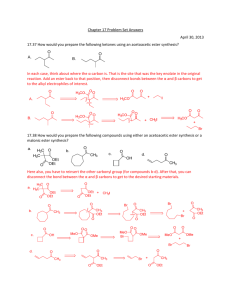
Spring 2005 Workshop #8 Problem #1: Synthesize the following target molecule starting from benzene and any simple four carbon (or less) units available through the Aldrich catalog. These can be your only sources of carbon atoms. Assume all other common reagents are readily available. Be sure to discuss strategic bond disconnections/retrosynthesis before you assemble the target structure. Problem #2: A good example of a classic crossed aldol reaction involves 2-butanone and acetone (we saw this in class). However, we encountered a problem with this reaction in that MULTIPLE acidic protons are present in each of these starting materials ( to the carbonyl). Consequently, we can envision that there are a variety of combinations of enolate (nucleophile) and electrophilic carbonyls that could lead to a whole mess of products. In this problem, you will carry out a crossed aldol condensation (assume acidic reaction conditions) between butanal and ethyl phenyl ketone. (a) Identify each of the possible nucleophile (enolate) and electrophile combinations and the product structures that their interactions generate by completing the table below (be careful of E vs. Z stereochemistry for any alkene containing products). Nucleophile (enolate) Electrophile Expected Product(s) (b) Splitting up the responsibility in your workshop group, show the complete mechanism of reaction for any ONE of the products and discuss the general features (as a group) that pertain to the aldol mechanism. Remember, the reaction is catalyzed by ACID! (c) Certainly, this reaction in its present from leads to a mess of products. So, it is appropriate to consider a Claisen-Schmidt modification to lead to greater product selectivity. Suggest structural variations in butanal and ethyl phenyl ketone that will reduce the total number of products. (d) In class, we said that the initial aldol product that is formed, subsequently eliminates water to yield an ,-unsaturated carbonyl compound. Surely, this is true either under acidic or basic conditions. However, under basic conditions this elimination of water reaction is not as favorable from an equilibrium perspective and requires higher temperatures or excessive quantities of base to promote the elimination. For example, the Keq >> 1 under acidic conditions, but Keq = 1 under basic conditions. Explain this discrepancy. Problem #3: Provide a complete reaction mechanism to show how the following aldol-type conversion occurs in the presence of NaOH. Be sure to explain why only the first two products form and why product #1 is the MAJOR product. Problem #4: If we react 1 equivalent of acetone with 2 equivalents of benzaldehyde in an aldol-type reaction in the presence of NaOH, an unexpected product forms called dibenzalacetone: C17H14O; 1HNMR (400 MHz, CDCl3) 7.35 (m, 10H), 7.1 (d, 2H, J = 15 Hz), 6.3 (d, 2H, J = 15 Hz); Offresonance decoupled 13C-NMR (400 MHz, CDCl3) 220 (s), 153 (s), 148 (d), 140 (d), 136 (d), 112 (d), 107 (d). Try to come up with the structure of dibenzalacetone using the spectroscopic data (think symmetry!) and show mechanistically how it forms. Problem #5: Synthesize the following target molecule starting from benzene and any simple four carbon units or less. NOTE: you must make the C=C via aldol chemistry and not by using Wittig chemistry. Be sure to discuss strategic bond disconnections/retrosynthesis before you assemble the target structure.