problem set 3
advertisement

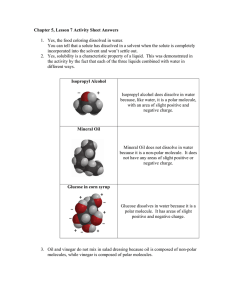
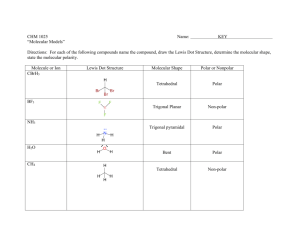
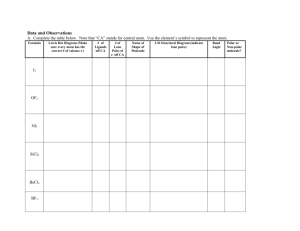
Chem 111 Molecules & Their Shapes 1. Use VSEPR theory to predict the shape of the following and draw a 3dimensional depiction of each: 2. Three different structures (not resonance structures) can be drawn for C2H2Cl2. Draw them and indicate whether each is polar or non-polar. 3. TeCl4 has a dipole moment. Why does this rule out tetrahedron and square planar as possible shapes for the molecule? With Te as the central atom and Cl placed in either a tetrahedral or square planar arrangement around it, the molecule would have to be non-polar. Since it is polar, it can't adopt either of these shapes. (The molecule is see-saw shaped.) 4. Arrange the following molecules in order of increasing polarity: H2O, H2Se, H2Te, H2S H2 O > H2S > H2 Se > H2Te 5. Draw a 3-dimensional depiction of each of the following molecules and indicate whether it is polar or non-polar: