Name: Date: Performances of Understanding:
advertisement

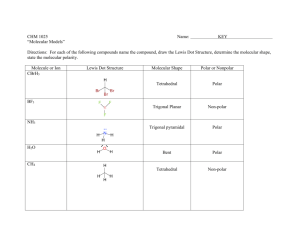
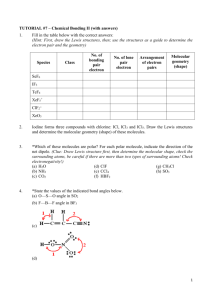
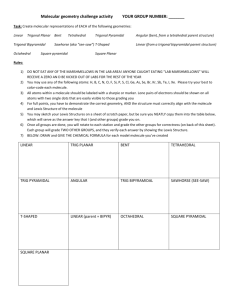
Name: _______________________ Date: _______________________ Learning Target: I can describe the postulates of kinetic molecular theory. Criteria for Success: I can describe the kinetic molecular theory (KMT). I can explain how the kinetic molecular theory applies to gases. I can define an ideal gas and a real gas. Performances of Understanding: -I will listen attentively while taking written notes in Cornell format about the kinetic molecular theory. -I will model the movement of gas particles using different size shot. -I will self-assess my understanding of the learning target using my Qwizdom remote. Notes Use this section of your notes to write questions, make connections, and sketch what you are visualizing! The Kinetic-Molecular Theory of Gases A. The kinetic-molecular theory is based on the idea that particles of matter are always in ___________. For gases, this theory is based on the following _______ assumptions. 1. Gases consist of __________ numbers of tiny particles that are ______ _________ relative to their size. Most of the volume of a gas is _______________ _____________. 2. Collisions between gas particles and between particles and container walls are __________________ collisions. a. An ________________ collision is one in which there is no net loss of energy. 3. Gas particles are in __________________, rapid, random ______________. a. Gases therefore posses ____________ energy which is the energy of ______________. 4. There are no forces of ____________________ or ___________________ between gas particles. 5. The average kinetic energy of gas particles depends on __________________. a. Kinetic energy is given by the following equation: KE= ½ mv2 B. An ____________ gas is an imaginary gas that perfectly fits all the assumptions of the kinetic-molecular theory. C. A __________ gas is a gas that does not behave completely according to the assumptions of the kinetic-molecular theory. The more __________ the gas molecules the greater the deviation from ideal behavior. The Kinetic-Molecular Theory of Gases and the Nature of Gases A. ___________________. Gases do not have a definite ___________ or a definite ___________. They completely fill their container. B. ____________________. Because the _______________ forces between gases is insignificant, gas particles can easily slide past one another. C. _____ ________________. The gas particles are very far apart in the gaseous state. D. ______________________. During ________________________ particles that were very far apart are crowded together. E. _____________________ and ______________________. 1. Spontaneous mixing of the particles of two substances caused by their random motion is called _____________________. 2. ____________________ is a process by which gas particles pass through a tiny opening. ¿Qué Dijo? Unit Ten 1 Guided/Independent Practice Gas He O2 H2 H2O N2 HCl NH3 Lewis Structure Molecular Geometry 1. A. Linear B. Bent C. Trigonal Planar D. Trigonal Pyramidal E. Tetrahedral F. Undefined 4. A. Linear B. Bent C. Trigonal Planar D. Trigonal Pyramidal E. Tetrahedral F. Undefined 7. A. Linear B. Bent C. Trigonal Planar D. Trigonal Pyramidal E. Tetrahedral F. Undefined 10. A. Linear B. Bent C. Trigonal Planar D. Trigonal Pyramidal E. Tetrahedral F. Undefined 13. A. Linear B. Bent C. Trigonal Planar D. Trigonal Pyramidal E. Tetrahedral F. Undefined 16. A. Linear B. Bent C. Trigonal Planar D. Trigonal Pyramidal E. Tetrahedral F. Undefined 19. A. Linear B. Bent C. Trigonal Planar D. Trigonal Pyramidal E. Tetrahedral F. Undefined Unit Ten 2 Polar or NonPolar 2. A. Polar B. Non-Polar Significant deviation from ideal behavior? Explain 3. Yes or No 5. A. Polar B. Non-Polar 6. 8. A. Polar B. Non-Polar 9. 11. A. Polar B. Non-Polar 12. 14. A. Polar B. Non-Polar 15. 17. A. Polar B. Non-Polar 18. 20. A. Polar B. Non-Polar 21. Yes or No Yes or No Yes or No Yes or No Yes or No Yes or No