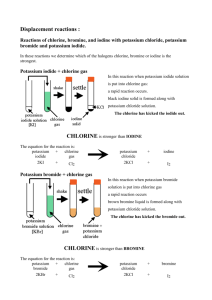
Reactivity of the
Halogens
This worksheet is designed to be completed alongside the lesson.
The icons on the left tell you which pages of the lesson the questions relate to.
Name:
Date:
The halogens all react with other substances in a similar way. However, some
halogens are more reactive than others. This lesson will help you find an order of
reactivity for the halogens.
1
2
1.
2.
3.
a.
What colours are chlorine, bromine and iodine when dissolved in
water?
b.
What colours are chlorine, bromine and iodine when dissolved in
hexane?
When potassium iodide reacts with chlorine solution, the colour
changes.
a.
What colour first appears on mixing?
b.
What substance could this be? How does adding hexane confirm
your answer?
The word equation for the reaction that has happened is:
potassium iodide + chlorine potassium chloride + iodine
a.
What does this tell you about the reactivity of chlorine compared
to iodine? Explain your answer.
b.
Would there be any reaction when chlorine is added to potassium
chloride solution? Why do you think this is?
c.
Would there be any reaction when bromine is added to potassium
chloride solution? Explain your answer.
1
Version 2.0
Copyright © 2005 PLATO Learning (UK) Ltd. All rights reserved.
Reactivity of the
Halogens
–
3
4.
5
The table below summarises the products formed when a halogen
dissolved in water is mixed with a halogen compound in solution.
Potassium
chloride
Potassium
bromide
Chlorine
Potassium
iodide
iodine + potassium
chloride
Bromine
Iodine
6
–
8
5.
a.
Complete the table using the information in the videos. Write ‘no
reaction’ if nothing happens.
b.
Use this table to order chlorine, bromine and iodine from most
reactive to least reactive. Explain how you arrived at your answer.
c.
Do you think fluorine would be more or less reactive than
chlorine? Explain your answer.
d.
Do you think astatine would be more or less reactive than iodine?
Explain your answer.
a.
Which reacts most vigorously with iron, chlorine or bromine?
b.
Which reacts most vigorously with aluminium, iodine or fluorine?
c.
Which reacts most vigorously with magnesium, chlorine or
fluorine?
d.
What evidence is there in the videos to support your answers?
2
Version 2.0
Copyright © 2005 PLATO Learning (UK) Ltd. All rights reserved.




![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)