(2)
advertisement
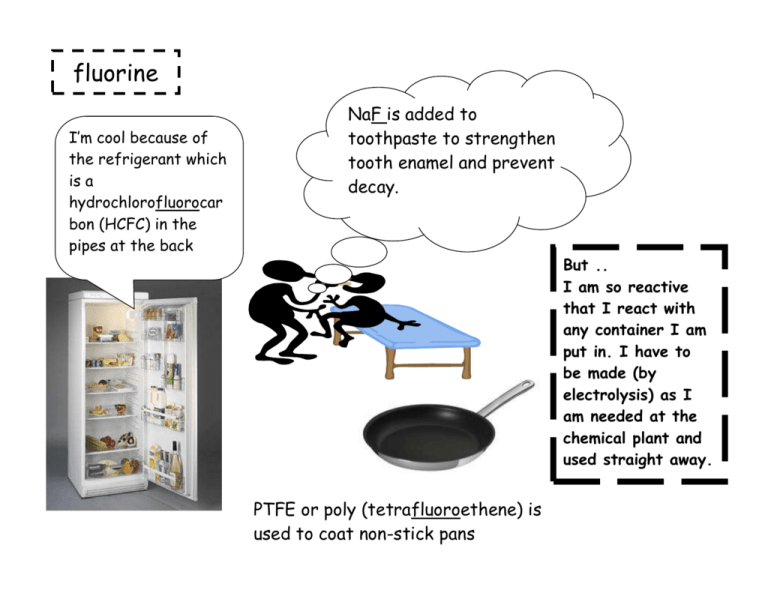
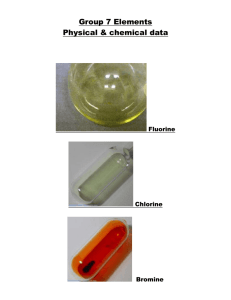
fluorine I’m cool because of the refrigerant which is a hydrochlorofluorocar bon (HCFC) in the pipes at the back NaF is added to toothpaste to strengthen tooth enamel and prevent decay. But .. I am so reactive that I react with any container I am put in. I have to be made (by electrolysis) as I am needed at the chemical plant and used straight away. PTFE or poly (tetrafluoroethene) is used to coat non-stick pans fluorine F F Fluorine is the most abundant halogen in the earth along with chlorine. It is always found in a compound. It has a low melting point and boiling point, the lowest of the halogens. This is because the instantaneous dipole-induced dipole bonds are weak due to F2 having less electrons than any other halogen molecules. It is a pale yellow gas. Fluorine is the most electronegative element of all. It is the most reactive halogen, reactivity decreases down the group and fluorine is at the top of the group. It will always have the oxidation state -1. This means that in FCl, fluorine is -1 and chlorine is +1. chlorine PVC or poly(vinylchloride) needs chlorine for its manufacture I am often made at the plant where brine is electrolysed to cut down on the transport of chlorine. Both reactants are products of the electrolysis. 2NaOH + Cl2 NaCl + NaClO + H2O I’m glad this swimming pool has a small amount of chlorine in to kill bacteria! CFCs (chlorofluorocarbons) are banned now but they are still destroying the ozone layer. chlorine Chlorine is the most abundant halogen in the earth along with chlorine. It is always found in a compound. It has a low melting point and boiling point, second lowest to fluorine. This is because the instantaneous dipole-induced dipole bonds are weak due to Cl2 having less electrons than bromine and iodine molecules. It is a pale green gas. It is the second most reactive halogen since reactivity decreases down the group. It will usually have the oxidation state -1. Exceptions are when bonded to F (always -1) or O (would be -2). bromine Tanker is lead lined and has a cage for protection I have protective clothing and breathing apparatus Bromomethane is a pesticide. They are trying to ban it because it destroys the ozone layer Bromine is used in the manufacture of medicines. I’m so glad that this sofa has a flame retardant manufactured using bromine. bromine Bromine is always found in a compound. It has a low melting point and boiling point although higher than fluorine and chlorine. This is because the instantaneous dipole-induced dipole bonds are weak but Br2 has more electrons than chlorine and fluorine. It is a dark red volatile liquid. It is the third most reactive halogen since reactivity decreases down the group. It will usually have the oxidation state -1. Exceptions are when bonded to F or Cl (always -1) or O (would be -2). Iodine is safest to transport because it is a solid Iodine can be manufactured from sea weed. We need iodine in our diet to remain healthy. It comes from vegetables grown in soil containing iodide ions. I’m used in the manufactu re of medicines Swab the patient with the antiseptic iodine solution. iodine Iodine is always found in a compound. Its melting and boiling point are higher than fluorine, chlorine or bromine. This is because the instantaneous dipole-induced dipole bonds are stronger (it has more electrons). It is a shiny black solid and if you heat it up it sublimes to form a purple vapour. It is the fourth most reactive halogen since reactivity decreases down the group. It will usually have the oxidation state -1. Exceptions are when bonded to F, Cl or Br (always -1) or O (would be -2).



![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)






