GROUP VII ELEMENTS

U1 M3 Group VII elements page 1 of 4
GROUP VII ELEMENTS a) explain the variations in physical properties of the elements in terms of structure and bonding (refer to volatility, density, state, only a description of the colour is required) b) explain the relative reactivities of the elements as oxidising agents
(include reactions with sodium thiosulphate and refer to E ө
values, also use solutions of the elements with bleach, bromine water and iodine solution) c) describe the reactions of the elements with hydrogen d) explain the relative stabilities of the hydrides (include reference to bond energies) e) describe the reactions of the halide ions with:- (i) aqueous solution of
AgNO
3
followed by aqueous ammonia (ii) conc. sulphuric acid f) describe the reactions of chlorine with cold and with hot aqueous solution of sodium hydroxide (mention changes in oxidation number)
Physical properties of halogens
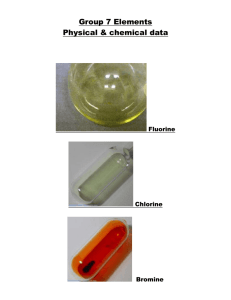
All the halogens exist as diatomic molecules at room temperature. Their melting and boiling points increase with increasing atomic number, because the dispersion forces (van der Waals forces) between the molecules become stronger as the number of electrons, and whereas fluorine and chlorine are normally gases, bromine is a liquid and iodine is a solid. The colour of the elements also deepens from almost colourless fluorine through yellow-green chlorine and red-brown to the deep, lustrous purple-black of iodine.
Reactivity of the halogens
Order of reactivity increases up the group.
All halogens act as oxidising agents. ½ X
(g)
+ e-
X -
(g)
+ (aq)
X -
(aq)
Strength as an oxidising agent is determined by 3 factors
1.
Bond dissociation (energy needed to break the molecule into 2 atoms)
2.
Electron affinities /kJ mol -1
3.
Hydration energy /kJ mol -1
The first is endothermic and the last two are exothermic processes.
U1 M3 Group VII elements page 2 of 4
The total energy change (kJ mol -1 ) from these three processes are:- fluorine -775 chlorine -607 bromine -580 iodine -541
Since the fluoride ion shows the most negative enthalpy, it is the most stable i.e. the most readily formed which translates to the most reactive halogen, while iodine shows the least negative enthalpy change and this means it is the least reactive halogen.
Reactions of the halogens with hydrogen and explanation of the trend of thermal stability of the hydrides
H
2(g)
+ X
2(g)
2HX
(g)
Fluorine :- it reacts with hydrogen gas explosively at room temperature
Chlorine:- it reacts with hydrogen explosively only after the initiation of the reaction by a spark or a flash of light
Bromine :- it only reacts in the presence of heat and a catalyst
Iodine :- even with the presence of heat and a catalyst, it is a reversible reaction with the product quickly decomposing to the starting materials
Thermal stability of the HX decreases down the group.
This can be easily explained by the trend of bond dissociation energy.
As the size of the halogen atom increases down the group, the degree of orbital overlap becomes less extensive and thus the bond strength decreases.
NB Acid strength increases down the group i.e. HI > HBr > HCl >> HF
U1 M3 Group VII elements page 3 of 4
Reaction of the halides
Table #1: Showing reaction of halides with aqueous ammonia and conc. sulphuric acid
Reagent F -
AgNO
3
NH
3(aq)
Cl Br -
No ppt White ppt Cream ppt
No reaction
Soluble in dilute
Soluble in conc. reagent
I -
Yellow ppt
Insoluble in dilute or conc. reagent reagent
Conc.
H
2
SO
4
HF(g) HCl (g) HBr (g) +
Br
2
(g)
Only I
2
(g)
Reaction of chlorine with NaOH
1.
With cold, dilute NaOH
(aq)
Cl
2(g)
+ 2OH -
(aq)
Cl -
(aq)
+ ClO -
(aq)
+ H
2
O
(l)
2.
With hot, concentrated NaOH (at 348K)
3Cl
2(g)
+ 6OH -
(aq)
5Cl -
(aq)
+ ClO
3
-
(aq)
+ 3H
2
O
(l)
In reaction one, chlorine forms the chloride ion and the chlorate(I) ion.
While in reaction 2, chlorine forms the chloride ion and the chlorate (V) ion. It is an example of disproportionation .
Worksheet
U1 M3 Group VII elements page 4 of 4



![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)